当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural modifications of 2,3-indolobetulinic acid: Design and synthesis of highly potent α-glucosidase inhibitors.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-04-29 , DOI: 10.1016/j.bioorg.2019.102957 Elmira F Khusnutdinova 1 , Anastasiya V Petrova 2 , Ha Nguyen Thi Thu 3 , Anh Le Thi Tu 3 , Tra Nguyen Thanh 3 , Cham Ba Thi 3 , Denis A Babkov 4 , Oxana B Kazakova 1
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-04-29 , DOI: 10.1016/j.bioorg.2019.102957 Elmira F Khusnutdinova 1 , Anastasiya V Petrova 2 , Ha Nguyen Thi Thu 3 , Anh Le Thi Tu 3 , Tra Nguyen Thanh 3 , Cham Ba Thi 3 , Denis A Babkov 4 , Oxana B Kazakova 1
Affiliation
|
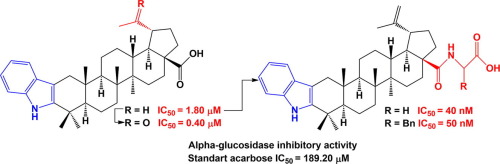
A series of nineteen nitrogen-containing lupane triterpenoids was obtained by modification of C2, C3, C20 and C28 positions of betulonic acid and their α-glucosidase inhibiting activity was investigated. Being a leader compound from our previous study, 2,3-indolo-betulinic acid was used as the main template for different modifications at C-(28)-carboxyl group to obtain cyano-, methylcyanoethoxy-, propargyloxy- and carboxamide derivatives. 20-Oxo- and 29-hydroxy-20-oxo-30-nor-analogues of 2,3-indolo-betulinic acid were synthesized by ozonolysis of betulonic acid followed by Fischer indolization reaction. To compare the influence of the fused indole or the seven-membered A-ring on the inhibitory activity, lupane A-azepanones with different substituents at C28 were synthesized. The structure-activity relationships revealed that the enzyme inhibition activity dramatically increased (up to 4730 times) when the carboxylic group of 2,3-indolo-betulinic acid was converted to the corresponding amide. Thus, the IC50 values for glycine amide and L-phenylalanine amides were 0.04 and 0.05 μM, respectively. This study also revealed that 2,3-indolo-platanic acid is 4.5 times more active than the parent triterpenoid with IC50 of 0.4 μM. Molecular modeling suggested that improved potency is due to additional polar interactions formed between C28 side chain and a sub-pocket of the α-glucosidase allosteric site.
中文翻译:

2,3-吲哚贝酸的结构修饰:高效α-葡萄糖苷酶抑制剂的设计和合成。
通过修饰丁二酸的C2,C3,C20和C28位置,获得了一系列十九个含氮的羽扇豆三萜类化合物,并研究了它们对α-葡萄糖苷酶的抑制活性。作为我们先前研究的先导化合物,将2,3-吲哚-布丁酸用作主要模板,对C-(28)-羧基进行不同的修饰,以获得氰基-,甲基氰基乙氧基-,炔丙基氧基-和羧酰胺衍生物。通过对丁二酸进行臭氧分解,然后进行费歇尔吲哚化反应,合成了2,3-吲哚-丁二酸的20-羟基和29-羟基-20-羰基-30-正类似物。为了比较稠合的吲哚或七元A环对抑制活性的影响,合成了在C28处具有不同取代基的卢潘烷A-氮杂环庚烷酮。结构-活性关系表明,当2,3-吲哚-贝多酸的羧酸基团转化为相应的酰胺时,酶的抑制活性急剧增加(高达4730倍)。因此,甘氨酸酰胺和L-苯丙氨酸酰胺的IC50值分别为0.04和0.05μM。该研究还表明,2,3-吲哚-铂酸的活性是母体三萜类化合物的4.5倍,IC50为0.4μM。分子模型表明,效力的提高是由于在C28侧链和α-葡萄糖苷酶变构位点的一个亚口袋之间形成了额外的极性相互作用。分别为04和0.05μM。该研究还表明,2,3-吲哚-铂酸的活性是母体三萜类化合物的4.5倍,IC50为0.4μM。分子模型表明,效力的提高是由于在C28侧链和α-葡萄糖苷酶变构位点的一个亚口袋之间形成了额外的极性相互作用。分别为04和0.05μM。该研究还表明,2,3-吲哚-铂酸的活性是母体三萜类化合物的4.5倍,IC50为0.4μM。分子模型表明,效力的提高是由于在C28侧链和α-葡萄糖苷酶变构位点的一个亚口袋之间形成了额外的极性相互作用。
更新日期:2019-04-29
中文翻译:

2,3-吲哚贝酸的结构修饰:高效α-葡萄糖苷酶抑制剂的设计和合成。
通过修饰丁二酸的C2,C3,C20和C28位置,获得了一系列十九个含氮的羽扇豆三萜类化合物,并研究了它们对α-葡萄糖苷酶的抑制活性。作为我们先前研究的先导化合物,将2,3-吲哚-布丁酸用作主要模板,对C-(28)-羧基进行不同的修饰,以获得氰基-,甲基氰基乙氧基-,炔丙基氧基-和羧酰胺衍生物。通过对丁二酸进行臭氧分解,然后进行费歇尔吲哚化反应,合成了2,3-吲哚-丁二酸的20-羟基和29-羟基-20-羰基-30-正类似物。为了比较稠合的吲哚或七元A环对抑制活性的影响,合成了在C28处具有不同取代基的卢潘烷A-氮杂环庚烷酮。结构-活性关系表明,当2,3-吲哚-贝多酸的羧酸基团转化为相应的酰胺时,酶的抑制活性急剧增加(高达4730倍)。因此,甘氨酸酰胺和L-苯丙氨酸酰胺的IC50值分别为0.04和0.05μM。该研究还表明,2,3-吲哚-铂酸的活性是母体三萜类化合物的4.5倍,IC50为0.4μM。分子模型表明,效力的提高是由于在C28侧链和α-葡萄糖苷酶变构位点的一个亚口袋之间形成了额外的极性相互作用。分别为04和0.05μM。该研究还表明,2,3-吲哚-铂酸的活性是母体三萜类化合物的4.5倍,IC50为0.4μM。分子模型表明,效力的提高是由于在C28侧链和α-葡萄糖苷酶变构位点的一个亚口袋之间形成了额外的极性相互作用。分别为04和0.05μM。该研究还表明,2,3-吲哚-铂酸的活性是母体三萜类化合物的4.5倍,IC50为0.4μM。分子模型表明,效力的提高是由于在C28侧链和α-葡萄糖苷酶变构位点的一个亚口袋之间形成了额外的极性相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号