当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In-Situ Synthesis of Petal-Like MoO2@MoN/NF Heterojunction As Both an Advanced Binder-Free Anode and an Electrocatalyst for Lithium Ion Batteries and Water Splitting
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-04-26 00:00:00 , DOI: 10.1021/acssuschemeng.8b06321 Yan Sun 1 , Yinlong Zhou 1 , Yaping Zhu 1 , Yuhua Shen 1 , Anjian Xie 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-04-26 00:00:00 , DOI: 10.1021/acssuschemeng.8b06321 Yan Sun 1 , Yinlong Zhou 1 , Yaping Zhu 1 , Yuhua Shen 1 , Anjian Xie 1
Affiliation
|
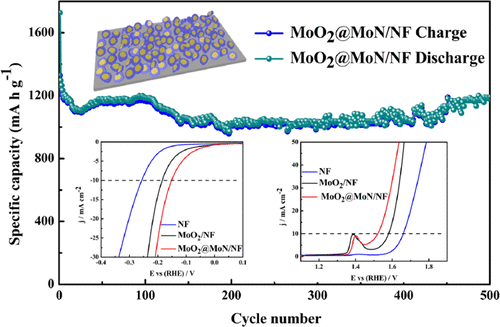
The design and synthesis of a heterostructure as well as binder-free electrodes or electrocatalysts with a porous nanostructure for enhancing electrochemical performance of lithium ion batteries and water splitting are still significant challenges to scientists. Here, for the first time, an in-situ synthesis of the porous petal-like MoN nanolayer-coated MoO2 heterojunction (MoO2@MoN) on commercial nickel foam (NF) by a localized nitrided transformation method as both a binder-free electrode for LIBs and an electrocatalyst for water splitting was performed. The in-situ formation of the MoN nanolayer could create a MoO2@MoN heterostructure, therefore enhancing the electronic conductivity and the electron/ion transfer. The XRD and XPS measurements confirmed the wonderful reversibility of the MoN layer during lithiation/delithiation cycling, which effectively promoted the long-life cycling performance (1190.1 mA h g–1 after 500 cycles at the current density of 0.5 A g–1). Meanwhile, the MoO2@MoN/NF/LiFePO4 full cell displayed stable capacity after 100 cycles. Moreover, the product also showed the improved electrocatalytic activity for hydrogen evolution and oxygen evolution. The excellent results suggest that our work opens a simple in-situ heterojunction formation pathway for the synthesis of other multifunctional materials applied in energy conversion and storage.
中文翻译:

花瓣状MoO 2 @ MoN / NF异质结的原位合成,同时用作高级无粘结剂阳极和锂离子电池及水分解的电催化剂
设计和合成异质结构以及具有多孔纳米结构的无粘合剂电极或电催化剂以增强锂离子电池的电化学性能和水分解仍然是科学家面临的重大挑战。在这里,首次通过局部氮化转化法在无粘结剂的情况下,通过局部氮化转化法在工业镍泡沫(NF)上原位合成了多孔花瓣状MoN纳米层包覆的MoO 2异质结(MoO 2 @MoN)。进行了用于LIB的电极和用于水分解的电催化剂。MoN纳米层的原位形成可能产生MoO 2MoN异质结构,因此增强了电子电导率和电子/离子转移。XRD和XPS测量锂化/脱锂循环,从而有效地促进了长寿命的循环性能期间证实了MON层的精彩可逆性(1190.1毫安汞柱-1在0.5 A G的电流密度500次循环后-1)。同时,MoO 2 @ MoN / NF / LiFePO 4100个循环后,满电池显示稳定的容量。此外,该产物还显示出改进的对氢析出和氧析出的电催化活性。优异的结果表明,我们的工作为合成用于能量转换和存储的其他多功能材料开辟了一条简单的原位异质结形成途径。
更新日期:2019-04-26
中文翻译:

花瓣状MoO 2 @ MoN / NF异质结的原位合成,同时用作高级无粘结剂阳极和锂离子电池及水分解的电催化剂
设计和合成异质结构以及具有多孔纳米结构的无粘合剂电极或电催化剂以增强锂离子电池的电化学性能和水分解仍然是科学家面临的重大挑战。在这里,首次通过局部氮化转化法在无粘结剂的情况下,通过局部氮化转化法在工业镍泡沫(NF)上原位合成了多孔花瓣状MoN纳米层包覆的MoO 2异质结(MoO 2 @MoN)。进行了用于LIB的电极和用于水分解的电催化剂。MoN纳米层的原位形成可能产生MoO 2MoN异质结构,因此增强了电子电导率和电子/离子转移。XRD和XPS测量锂化/脱锂循环,从而有效地促进了长寿命的循环性能期间证实了MON层的精彩可逆性(1190.1毫安汞柱-1在0.5 A G的电流密度500次循环后-1)。同时,MoO 2 @ MoN / NF / LiFePO 4100个循环后,满电池显示稳定的容量。此外,该产物还显示出改进的对氢析出和氧析出的电催化活性。优异的结果表明,我们的工作为合成用于能量转换和存储的其他多功能材料开辟了一条简单的原位异质结形成途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号