当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Olefin Metathesis with Neutral and Cationic Molybdenum Imido Alkylidene N Heterocyclic Carbene Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-27 , DOI: 10.1021/jacs.9b02092
Katharina Herz , Maren Podewitz 1 , Laura Stöhr , Dongren Wang , Wolfgang Frey , Klaus R Liedl 1 , Suman Sen , Michael R Buchmeiser
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-27 , DOI: 10.1021/jacs.9b02092
Katharina Herz , Maren Podewitz 1 , Laura Stöhr , Dongren Wang , Wolfgang Frey , Klaus R Liedl 1 , Suman Sen , Michael R Buchmeiser
Affiliation
|
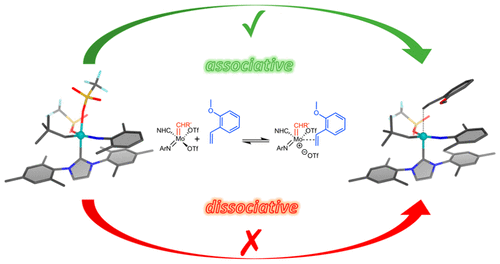
A series of neutral molybdenum imido alkylidene N-heterocyclic carbene (NHC) bistriflate and monotriflate monoalkoxide complexes as well as cationic molybdenum imido alkylidene triflate complexes have been subjected to NMR spectroscopic, X-ray crystallographic, and reaction kinetic measurements in order to gain a comprehensive understanding about the underlying mechanism in olefin metathesis of this new type of catalysts. On the basis of experimental evidence and on DFT calculations (BP86/def2-TZVP/D3/cosmo) for the entire mechanism, olefinic substrates coordinate trans to the NHC of neutral 16-electron complexes via an associative mechanism, followed by dissociation of an anionic ligand (e.g., triflate) and formation of an intermediary molybdacyclobutane trans to the NHC. Formation of a cationic complex is crucial in order to become olefin metathesis active. Variations in the NHC, the imido, the alkoxide, and the noncoordinating anion revealed their influence on reactivity. The reaction of neutral 16-electron complexes with 2-methoxystyrene is faster for catalysts bearing one triflate and one fluorinated alkoxide than for catalysts bearing two triflate ligands. This is also reflected by the Gibbs free energy values for the transition states, ΔG‡303, which are significantly lower for catalysts bearing only one triflate than for the corresponding bistriflate complexes. Reaction of a solvent-stabilized cationic molybdenum imido alkylidene N-heterocyclic carbene (NHC) monotriflate complex with 2-methoxystyrene proceeded via an associative mechanism too. Reaction rates of both solvent-free and solvent-stabilized cationic Mo imido alkylidene NHC catalysts with 2-methoxystyrene are controlled by the cross-metathesis step but not by adduct formation.
中文翻译:

中性和阳离子钼亚氨基亚烷基N杂环卡宾配合物的烯烃复分解机理
一系列中性钼酰亚胺亚烷基 N-杂环卡宾 (NHC) 双氟甲磺酸酯和单三氟甲磺酸酯单烷氧化物配合物以及阳离子钼酰亚胺亚烷基三氟甲磺酸酯配合物已进行 NMR 光谱、X 射线晶体学和反应动力学测量,以获得全面的了解这种新型催化剂在烯烃复分解中的潜在机制。根据实验证据和整个机制的 DFT 计算 (BP86/def2-TZVP/D3/cosmo),烯烃底物通过缔合机制将反式协调到中性 16 电子配合物的 NHC,然后解离阴离子配体(例如,三氟甲磺酸酯)并形成与 NHC 反式的中间钼环丁烷。阳离子络合物的形成对于成为烯烃复分解活性的关键。NHC、亚氨基、醇盐和非配位阴离子的变化揭示了它们对反应性的影响。对于带有一种三氟甲磺酸酯和一种氟化醇盐的催化剂,中性 16 电子配合物与 2-甲氧基苯乙烯的反应比带有两个三氟甲磺酸酯配体的催化剂更快。这也反映在过渡态的吉布斯自由能值 ΔG‡303 中,仅含有一种三氟甲磺酸酯的催化剂的吉布斯自由能值显着低于相应的双氟甲磺酸酯配合物。溶剂稳定的阳离子钼亚胺亚烷基 N-杂环卡宾 (NHC) 单三氟甲磺酸酯配合物与 2-甲氧基苯乙烯的反应也通过缔合机制进行。
更新日期:2019-04-27
中文翻译:

中性和阳离子钼亚氨基亚烷基N杂环卡宾配合物的烯烃复分解机理
一系列中性钼酰亚胺亚烷基 N-杂环卡宾 (NHC) 双氟甲磺酸酯和单三氟甲磺酸酯单烷氧化物配合物以及阳离子钼酰亚胺亚烷基三氟甲磺酸酯配合物已进行 NMR 光谱、X 射线晶体学和反应动力学测量,以获得全面的了解这种新型催化剂在烯烃复分解中的潜在机制。根据实验证据和整个机制的 DFT 计算 (BP86/def2-TZVP/D3/cosmo),烯烃底物通过缔合机制将反式协调到中性 16 电子配合物的 NHC,然后解离阴离子配体(例如,三氟甲磺酸酯)并形成与 NHC 反式的中间钼环丁烷。阳离子络合物的形成对于成为烯烃复分解活性的关键。NHC、亚氨基、醇盐和非配位阴离子的变化揭示了它们对反应性的影响。对于带有一种三氟甲磺酸酯和一种氟化醇盐的催化剂,中性 16 电子配合物与 2-甲氧基苯乙烯的反应比带有两个三氟甲磺酸酯配体的催化剂更快。这也反映在过渡态的吉布斯自由能值 ΔG‡303 中,仅含有一种三氟甲磺酸酯的催化剂的吉布斯自由能值显着低于相应的双氟甲磺酸酯配合物。溶剂稳定的阳离子钼亚胺亚烷基 N-杂环卡宾 (NHC) 单三氟甲磺酸酯配合物与 2-甲氧基苯乙烯的反应也通过缔合机制进行。


































 京公网安备 11010802027423号
京公网安备 11010802027423号