当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni-Catalyzed Suzuki-Miyaura Cross-Coupling of α-Oxo-Vinylsulfones to Prepare C-Aryl Glycals and Acyclic Vinyl Ethers
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-26 , DOI: 10.1021/jacs.9b02312 Liang Gong 1 , Hong-Bao Sun 1 , Li-Fan Deng 1 , Xia Zhang 1 , Jie Liu 1 , Shengyong Yang 1 , Dawen Niu 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-26 , DOI: 10.1021/jacs.9b02312 Liang Gong 1 , Hong-Bao Sun 1 , Li-Fan Deng 1 , Xia Zhang 1 , Jie Liu 1 , Shengyong Yang 1 , Dawen Niu 1
Affiliation
|
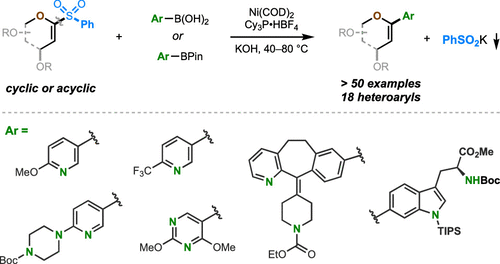
We demonstrate that readily available and bench-stable α-oxo-vinylsulfones are competent electrophiles in Ni-catalyzed Suzuki-Miyaura cross-coupling reactions. The C-sulfone bond in the α-oxo-vinylsulfone motif is cleaved chemoselectively in these reactions, furnishing C-aryl glycals or acyclic vinyl ethers in high yields. These reactions proceed under mild conditions and tolerate a remarkable scope of heterocycles and functional groups. Preliminary mechanistic studies revealed the importance of an α-heteroatom in facilitating these transformations.
中文翻译:

镍催化 Suzuki-Miyaura 交叉偶联 α-氧代-乙烯基砜制备 C-芳基乙二醇和无环乙烯基醚
我们证明,在 Ni 催化的 Suzuki-Miyaura 交叉偶联反应中,容易获得且工作台稳定的 α-氧代乙烯基砜是有能力的亲电试剂。在这些反应中,α-氧代-乙烯基砜基序中的 C-砜键被化学选择性地裂解,以高产率提供 C-芳基乙二醇或无环乙烯基醚。这些反应在温和的条件下进行,并且可以耐受范围广泛的杂环和官能团。初步的机理研究揭示了 α-杂原子在促进这些转变中的重要性。
更新日期:2019-04-26
中文翻译:

镍催化 Suzuki-Miyaura 交叉偶联 α-氧代-乙烯基砜制备 C-芳基乙二醇和无环乙烯基醚
我们证明,在 Ni 催化的 Suzuki-Miyaura 交叉偶联反应中,容易获得且工作台稳定的 α-氧代乙烯基砜是有能力的亲电试剂。在这些反应中,α-氧代-乙烯基砜基序中的 C-砜键被化学选择性地裂解,以高产率提供 C-芳基乙二醇或无环乙烯基醚。这些反应在温和的条件下进行,并且可以耐受范围广泛的杂环和官能团。初步的机理研究揭示了 α-杂原子在促进这些转变中的重要性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号