Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2019-04-27 , DOI: 10.1016/j.jmb.2019.04.026 Anwesha Sanyal 1 , Sayan Dutta 2 , Ali Camara 3 , Aswathy Chandran 2 , Antonius Koller 4 , Ben G Watson 3 , Ranjan Sengupta 3 , Daniel Ysselstein 2 , Paola Montenegro 2 , Jason Cannon 5 , Jean-Christophe Rochet 6 , Seema Mattoo 7
|
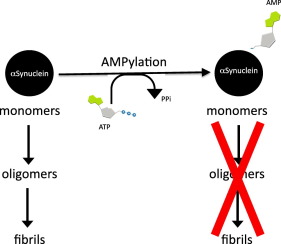
During disease, cells experience various stresses that manifest as an accumulation of misfolded proteins and eventually lead to cell death. To combat this stress, cells activate a pathway called unfolded protein response that functions to maintain endoplasmic reticulum (ER) homeostasis and determines cell fate. We recently reported a hitherto unknown mechanism of regulating ER stress via a novel post-translational modification called Fic-mediatedadenylylation/AMPylation. Specifically, we showed that the human Fic (filamentation induced by cAMP) protein, HYPE/FicD, catalyzes the addition of an adenosine monophosphate (AMP) to the ER chaperone, BiP, to alter the cell's unfolded protein response-mediated response to misfolded proteins. Here, we report that we have now identified a second target for HYPE—alpha-synuclein (αSyn), a presynaptic protein involved in Parkinson's disease. Aggregated αSyn has been shown to induce ER stress and elicit neurotoxicity in Parkinson's disease models. We show that HYPE adenylylates αSyn and reduces phenotypes associated with αSyn aggregation invitro, suggesting a possible mechanism by which cells cope with αSyn toxicity.
中文翻译:

α-突触核蛋白是Fic介导的腺苷酸化/ AMPylation的目标:对帕金森氏病的可能影响。
在疾病期间,细胞会经受各种压力,这些压力表现为错误折叠的蛋白质的积累,并最终导致细胞死亡。为了应对这种压力,细胞激活了一种称为未折叠蛋白反应的途径,该途径可维持内质网(ER)稳态并决定细胞命运。我们最近报道了一种迄今未知的通过称为Fic介导的腺苷酰化/ AMPylation的新型翻译后修饰调节内质网应激的机制。具体来说,我们显示了人类Fic(由cAMP诱导的细丝化)蛋白HYPE / FicD催化向ER伴侣BiP中添加单磷酸腺苷(AMP),从而改变了细胞对未折叠蛋白的未折叠蛋白响应介导的响应。在这里,我们报告说,我们现在已经确定了HYPE的第二个目标-α-突触核蛋白(αSyn),与帕金森氏病有关的突触前蛋白。在帕金森氏病模型中,聚集的αSyn已被证明可诱导内质网应激并引起神经毒性。我们显示HYPE腺苷酸化αSyn并减少与αSyn聚集相关的表型体外研究,提示细胞应对αSyn毒性的可能机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号