European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-04-28 , DOI: 10.1016/j.ejmech.2019.04.067 Srikanth Gatadi , Jitendra Gour , Manjulika Shukla , Grace Kaul , Arunava Dasgupta , Y.V. Madhavi , Sidharth Chopra , Srinivas Nanduri
|
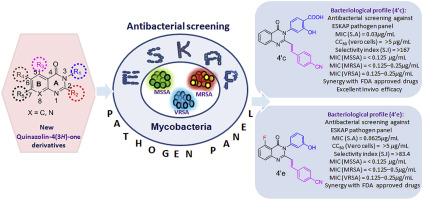
Staphylococcus aureus and Mycobacterium tuberculosis are major causative agents responsible for serious nosocomial and community-acquired infections impacting healthcare systems globally. Over several decades, these pathogens have developed resistance to multiple antibiotics significantly affecting morbidity and mortality. Thus, these recalcitrant pathogens are amongst the most formidable microbial pathogens for which international healthcare agencies have mandated active identification and development of new antibacterial agents for chemotherapeutic intervention. In our present work, a series of new quinazolin-4(3H)-one derivatives were designed, synthesized and evaluated for their antibacterial activity against ESKAP pathogens and pathogenic mycobacteria. The experiments revealed that 4'c, 4'e, 4'f and 4'h displayed selective and potent inhibitory activity against Staphylococcus aureus with MIC values ranging from 0.03-0.25 μg/mL. Furthermore, compounds 4'c and 4'e were found to be benign to Vero cells (CC50 = >5 μg/mL) and displayed promising selectivity index (SI) > 167 and > 83.4 respectively. Additionally, 4'c and 4'e demonstrated equipotent MIC against multiple drug-resistant strains of S. aureus including VRSA, concentration dependent bactericidal activity against S. aureus and synergized with FDA approved drugs. Moreover, compound 4′c exhibited more potent activity in reducing the biofilm and exhibited a PAE of ∼2 h at 10X MIC which is comparable to levofloxacin and vancomycin. In vivo efficacy of 4'c in murine neutropenic thigh infection model revealed that 4'c caused a similar reduction in cfu as vancomycin. Gratifyingly, compounds 4d, 4e, 9a, 9b, 14a, 4'e and 4'f also exhibited anti-mycobacterial activity with MIC values in the range of 2-16 μg/mL. In addition, the compounds were found to be less toxic to Vero cells (CC50 = 12.5->100 μg/mL), thus displaying a favourable selectivity index. The interesting results obtained here suggest the potential utilization of these new quinazolin-4(3H)-one derivatives as promising antibacterial agents for treating MDR-Staphylococcal and mycobacterial infections.
中文翻译:

新的喹唑啉-4(3 H)-one衍生物的合成和评价作为对多药耐药金黄色葡萄球菌和结核分枝杆菌的有效抗菌剂
金黄色葡萄球菌和结核分枝杆菌是导致全球范围内严重影响医院和社区的严重感染的主要病原体。几十年来,这些病原体已经对多种抗生素产生了抗药性,从而极大地影响了发病率和死亡率。因此,这些顽固性病原体是最强大的微生物病原体之一,国际医疗机构已要求其积极鉴定和开发用于化学干预的新型抗菌剂。在我们目前的工作中,一系列新的喹唑啉4(3 H设计,合成并评估了一种衍生物对ESKAP病原体和致病性分枝杆菌的抗菌活性。该实验表明4'c,4'e,4'f和4'H显示选择性的和有效的抑制活性对金黄色葡萄球菌与MIC值范围为0.03-0.25 μ克/毫升。此外,化合物4'c和4'e被认为是良性到Vero细胞(CC 50 => 5 μ克/毫升)并显示分别有前途的选择性指数(SI)> 167和> 83.4。此外,4'c和4'e针对多重耐药菌株表现出等效的MIC的金黄色葡萄球菌,包括VRSA,针对浓度依赖性杀菌活性的金黄色葡萄球菌和与FDA批准的协同作用的药物。此外,化合物4'c在还原生物膜方面表现出更强的活性,在10X MIC下的PAE约为2小时,与左氧氟沙星和万古霉素相当。4'c在鼠中性粒细胞减少大腿感染模型中的体内功效显示,4'c与万古霉素引起的cfu降低相似。令人欣慰的是,化合物4d,4e,9a,9b,14a,4'e和4'f在2-16的范围内也表现出抗分枝杆菌活性,MIC值 μ克/毫升。此外,发现化合物是有毒的Vero细胞更少(CC 50 = 12.5-14> 100 μ克/毫升),从而显示了良好的选择性指数。此处获得的有趣结果表明,这些新的quinazolin-4(3 H)-one衍生物作为潜在的抗菌剂可用于治疗MDR-葡萄球菌和分枝杆菌感染。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号