当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
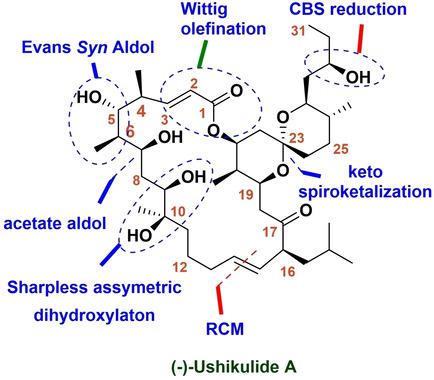
Progress towards the Synthesis of (‐)‐Ushikulide A: Synthesis of C1‐C15 Aliphatic and C17‐C31 Spiroketal Fragments by an Aldol Approach
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-04-26 , DOI: 10.1002/slct.201900252
Mohammad Ataur Rahman 1, 2 , N. Mallikarjuna Reddy 1 , Jhillu Singh Yadav 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2019-04-26 , DOI: 10.1002/slct.201900252
Mohammad Ataur Rahman 1, 2 , N. Mallikarjuna Reddy 1 , Jhillu Singh Yadav 1
Affiliation
|
A convergent approach towards the total synthesis of an immunosuppressant (‐)‐ushikulide A, has been described. Herein, we disclose the synthesis of the C1‐C15 linear fragment and the C17‐C31 spiroketal fragment of (‐)‐ushikulide A by the aldol approach. The C1‐C15 aliphatic fragment was synthesized by applying Evans syn aldol, acetate aldol reaction and the C17‐C31 spiroketal fragment of (‐)‐ushikulide A was achieved via non‐Evans syn aldol as well as spiroketalization reaction. The precursors for the spiroketalization reaction were synthesized from a common intermediate, derived from the non‐Evans syn aldol reaction.
中文翻译:

合成(‐)‐ Ushikulide A的进展:通过Aldol方法合成C1-C15脂族和C17-C31螺环片段
已经描述了一种收敛方法,可用于免疫抑制剂(-)-ushikulide A的总合成。在本文中,我们公开了通过醇醛缩合法合成(-)-ushikulide A的C1-C15线性片段和C17-C31螺环片段的合成方法。C1-C15脂肪族片段是通过应用Evans合成羟醛,乙酸羟醛反应合成的,(-)-ushikulide A的C17-C31螺旋酮化片段是通过非Evans合成羟醛和螺酮化反应获得的。螺环缩合反应的前体是从非埃文斯合成醇醛缩合反应衍生的通用中间体合成的。
更新日期:2019-04-26
中文翻译:

合成(‐)‐ Ushikulide A的进展:通过Aldol方法合成C1-C15脂族和C17-C31螺环片段
已经描述了一种收敛方法,可用于免疫抑制剂(-)-ushikulide A的总合成。在本文中,我们公开了通过醇醛缩合法合成(-)-ushikulide A的C1-C15线性片段和C17-C31螺环片段的合成方法。C1-C15脂肪族片段是通过应用Evans合成羟醛,乙酸羟醛反应合成的,(-)-ushikulide A的C17-C31螺旋酮化片段是通过非Evans合成羟醛和螺酮化反应获得的。螺环缩合反应的前体是从非埃文斯合成醇醛缩合反应衍生的通用中间体合成的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号