当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hydrogen-Bonding and Hydrophobic Groups Contribute Equally to the Binding of Hyperactive Antifreeze and Ice Nucleating Proteins to Ice
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-25 , DOI: 10.1021/jacs.9b02248 Arpa Hudait 1 , Yuqing Qiu 1 , Nathan Odendahl 1 , Valeria Molinero 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-25 , DOI: 10.1021/jacs.9b02248 Arpa Hudait 1 , Yuqing Qiu 1 , Nathan Odendahl 1 , Valeria Molinero 1
Affiliation
|
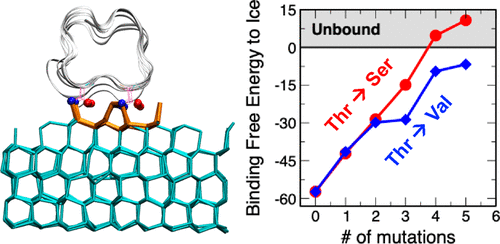
Hyperactive insect antifreeze proteins and bacterial ice-nucleating proteins are arguably the most potent ice-binding molecules in nature. These highly effective proteins bind ice through amphiphilic ice-binding sites based on arrays of threonine residues. It remains poorly understood how hydrophilic and hydrophobic groups of the binding site contribute to the ice affinity of proteins. Here, we use molecular simulations to demonstrate that the hydrogen-bonding and hydrophobic groups at the ice-binding site of the antifreeze protein TmAFP of Tenebrio molitor and extended ice-nucleating protein surfaces contribute distinctively yet almost equally in magnitude to their binding free energy to ice. The methyl groups rigidize the ice-binding site, slow the water dynamics at the ice-binding surface, and stabilize the clathrate-like water in the anchored clathrate motif that binds these proteins to ice. We find that hydrophobic dehydration of the methyl group does not contribute to the binding free energy of the protein to ice. The role of the hydroxyl groups is to anchor the clathrate-like water through direct hydrogen-bonding, positioning and slowing the dynamics of water at the trough of the binding site. We uncover a correlation between slower dynamics of water at the binding site for the protein in solution and stronger free energy of binding of the protein to ice. The synergy between hydrophobic and hydrophilic groups unveiled by this study provides guidance for the design of synthetic ice-binding molecules with enhanced ice nucleation and antifreeze activity.
中文翻译:

氢键和疏水基团同样有助于高活性防冻剂和冰核蛋白与冰的结合
过度活跃的昆虫抗冻蛋白和细菌冰核蛋白可以说是自然界中最有效的冰结合分子。这些高效蛋白质通过基于苏氨酸残基阵列的两亲性冰结合位点结合冰。结合位点的亲水和疏水基团如何影响蛋白质的冰亲和力仍然知之甚少。在这里,我们使用分子模拟来证明,黄粉虫的抗冻蛋白 TmAFP 和扩展的冰成核蛋白表面的冰结合位点处的氢键和疏水基团对它们的结合自由能的贡献显着但几乎相等。冰。甲基使冰结合位点变硬,减慢冰结合表面的水动力学,并稳定锚定的包合物基序中的包合物样水,将这些蛋白质与冰结合。我们发现甲基的疏水性脱水对蛋白质与冰的结合自由能没有贡献。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。我们发现甲基的疏水性脱水对蛋白质与冰的结合自由能没有贡献。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。我们发现甲基的疏水性脱水对蛋白质与冰的结合自由能没有贡献。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。
更新日期:2019-04-25
中文翻译:

氢键和疏水基团同样有助于高活性防冻剂和冰核蛋白与冰的结合
过度活跃的昆虫抗冻蛋白和细菌冰核蛋白可以说是自然界中最有效的冰结合分子。这些高效蛋白质通过基于苏氨酸残基阵列的两亲性冰结合位点结合冰。结合位点的亲水和疏水基团如何影响蛋白质的冰亲和力仍然知之甚少。在这里,我们使用分子模拟来证明,黄粉虫的抗冻蛋白 TmAFP 和扩展的冰成核蛋白表面的冰结合位点处的氢键和疏水基团对它们的结合自由能的贡献显着但几乎相等。冰。甲基使冰结合位点变硬,减慢冰结合表面的水动力学,并稳定锚定的包合物基序中的包合物样水,将这些蛋白质与冰结合。我们发现甲基的疏水性脱水对蛋白质与冰的结合自由能没有贡献。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。我们发现甲基的疏水性脱水对蛋白质与冰的结合自由能没有贡献。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。我们发现甲基的疏水性脱水对蛋白质与冰的结合自由能没有贡献。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。羟基的作用是通过直接的氢键结合来锚定类似包合物的水,在结合位点的低谷处定位和减缓水的动力学。我们揭示了溶液中蛋白质结合位点处水的较慢动力学与蛋白质与冰结合的更强自由能之间的相关性。该研究揭示的疏水基团和亲水基团之间的协同作用为设计具有增强冰成核和抗冻活性的合成冰结合分子提供了指导。































 京公网安备 11010802027423号
京公网安备 11010802027423号