当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis: a study based on a three-dimensional biomimetic environment.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-04-25 , DOI: 10.1038/s41419-019-1586-1 Guan Zheng 1 , Zhongyu Xie 1, 2 , Peng Wang 1, 2 , Jinteng Li 1 , Ming Li 1 , Shuizhong Cen 1 , Su'an Tang 1 , Wenjie Liu 1 , Guiwen Ye 1 , Yuxi Li 1 , Shan Wang 3 , Xiaohua Wu 3 , Hongjun Su 3 , Yanfeng Wu 3 , Huiyong Shen 1, 2
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-04-25 , DOI: 10.1038/s41419-019-1586-1 Guan Zheng 1 , Zhongyu Xie 1, 2 , Peng Wang 1, 2 , Jinteng Li 1 , Ming Li 1 , Shuizhong Cen 1 , Su'an Tang 1 , Wenjie Liu 1 , Guiwen Ye 1 , Yuxi Li 1 , Shan Wang 3 , Xiaohua Wu 3 , Hongjun Su 3 , Yanfeng Wu 3 , Huiyong Shen 1, 2
Affiliation
|
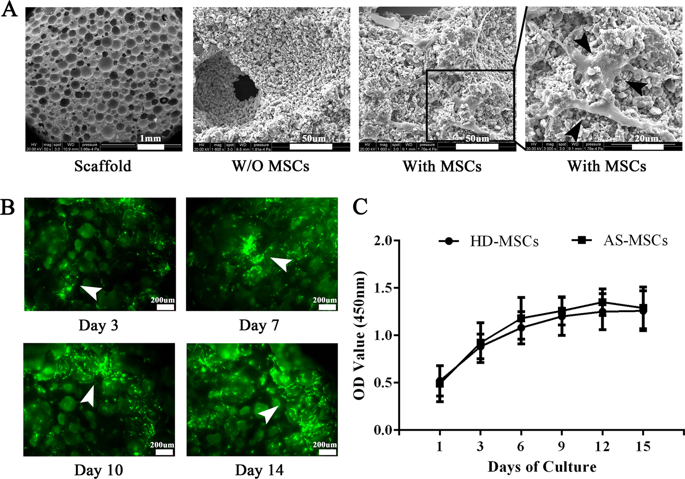
The mechanism of pathological osteogenesis in Ankylosing spondylitis (AS) is largely unknown. Our previous studies demonstrated that the imbalance between BMP-2 and Noggin secretion induces abnormal osteogenic differentiation of marrow-derived mesenchymal stem cells (MSCs) from AS patients in a two-dimensional culture environment. In this study, HA/β-TCP scaffolds were further used as a three-dimensional (3D) biomimetic culture system to mimic the bone microenvironment in vivo to determine the abnormal osteogenic differentiation of AS-MSCs. We demonstrated that when cultured in HA/β-TCP scaffolds, AS-MSCs had a stronger osteogenic differentiation capacity than that of MSCs from healthy donors (HD-MSCs) in vitro and in vivo. This dysfunction resulted from BMP2 overexpression in AS-MSCs, which excessively activated the Smad1/5/8 and ERK signalling pathways and finally led to enhanced osteogenic differentiation. Both the signalling pathway inhibitors and siRNAs inhibiting BMP2 expression could rectify the enhanced osteogenic differentiation of AS-MSCs. Furthermore, BMP2 expression in ossifying entheses was significantly higher in AS patients. In summary, our study demonstrated that AS-MSCs possess enhanced osteogenic differentiation in HA/β-TCP scaffolds as a 3D biomimetic microenvironment because of BMP2 overexpression, but not Noggin. These results provide insights into the mechanism of pathological osteogenesis, which can aid in the development of niche-targeting medications for AS.
中文翻译:

强直性脊柱炎中间充质干细胞的成骨分化增强:基于三维仿生环境的研究。
强直性脊柱炎(AS)的病理成骨机制尚不清楚。我们以前的研究表明,在二维培养环境中,BMP-2和Noggin分泌之间的不平衡诱导了AS患者骨髓来源的间充质干细胞(MSC)的异常成骨分化。在这项研究中,HA /β-TCP支架被进一步用作三维(3D)仿生培养系统,以在体内模拟骨骼微环境,以确定AS-MSC的异常成骨分化。我们证明,在HA /β-TCP支架中进行培养时,AS-MSC在体外和体内均比健康供体(HD-MSC)的MSC具有更强的成骨分化能力。这种功能障碍是由AS-MSC中BMP2过表达引起的,过度激活Smad1 / 5/8和ERK信号通路,最终导致成骨分化增强。信号通路抑制剂和抑制BMP2表达的siRNA均可纠正AS-MSC的成骨分化。此外,AS患者的骨化蛋白中的BMP2表达明显更高。总之,我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。信号通路抑制剂和抑制BMP2表达的siRNA均可纠正AS-MSC的成骨分化。此外,AS患者的骨化蛋白中的BMP2表达明显更高。总之,我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。信号通路抑制剂和抑制BMP2表达的siRNA均可纠正AS-MSC的成骨分化。此外,AS患者的骨化蛋白中的BMP2表达明显更高。总之,我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。
更新日期:2019-05-16
中文翻译:

强直性脊柱炎中间充质干细胞的成骨分化增强:基于三维仿生环境的研究。
强直性脊柱炎(AS)的病理成骨机制尚不清楚。我们以前的研究表明,在二维培养环境中,BMP-2和Noggin分泌之间的不平衡诱导了AS患者骨髓来源的间充质干细胞(MSC)的异常成骨分化。在这项研究中,HA /β-TCP支架被进一步用作三维(3D)仿生培养系统,以在体内模拟骨骼微环境,以确定AS-MSC的异常成骨分化。我们证明,在HA /β-TCP支架中进行培养时,AS-MSC在体外和体内均比健康供体(HD-MSC)的MSC具有更强的成骨分化能力。这种功能障碍是由AS-MSC中BMP2过表达引起的,过度激活Smad1 / 5/8和ERK信号通路,最终导致成骨分化增强。信号通路抑制剂和抑制BMP2表达的siRNA均可纠正AS-MSC的成骨分化。此外,AS患者的骨化蛋白中的BMP2表达明显更高。总之,我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。信号通路抑制剂和抑制BMP2表达的siRNA均可纠正AS-MSC的成骨分化。此外,AS患者的骨化蛋白中的BMP2表达明显更高。总之,我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。信号通路抑制剂和抑制BMP2表达的siRNA均可纠正AS-MSC的成骨分化。此外,AS患者的骨化蛋白中的BMP2表达明显更高。总之,我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。我们的研究表明,AS-MSC作为3D仿生微环境,在HA /β-TCP支架中具有增强的成骨分化能力,这是因为BMP2过表达,而Noggin则不然。这些结果为病理性成骨机制提供了见识,其可以帮助开发针对AS的利基靶向药物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号