当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Caged Cyclopropenes with Improved Tetrazine Ligation Kinetics
Organic Letters ( IF 4.9 ) Pub Date : 2019-04-24 00:00:00 , DOI: 10.1021/acs.orglett.9b01177 Pratik Kumar 1 , Omar Zainul 1 , Frank M. Camarda 1 , Ting Jiang 1 , John A. Mannone 1 , Wei Huang 1 , Scott T. Laughlin 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-04-24 00:00:00 , DOI: 10.1021/acs.orglett.9b01177 Pratik Kumar 1 , Omar Zainul 1 , Frank M. Camarda 1 , Ting Jiang 1 , John A. Mannone 1 , Wei Huang 1 , Scott T. Laughlin 1
Affiliation
|
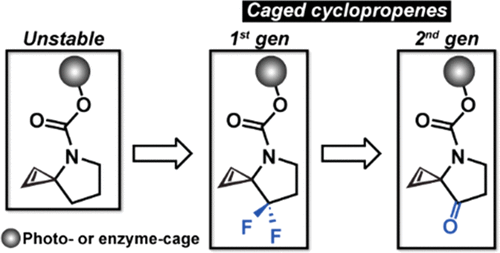
Activatable cyclopropenes are unreactive toward their inverse electron demand Diels–Alder reaction partner (e.g., s-tetrazines) until they are activated. The activation strategy is highly modular due to the cyclopropene’s ability to be caged by various light- and enzyme-activatable groups. This work describes the next generation of activatable cyclopropenes with a new core scaffold that maintains the activation modularity of the first generation but improves upon the ligation kinetics with s-tetrazines by ≤270-fold.
中文翻译:

笼型环丙烯具有改善的四嗪连接反应动力学
可活化的环丙烯对它们的逆电子需求Diels–Alder反应伙伴(例如,s-四嗪)不发生反应,直到被活化为止。由于环丙烯具有被各种可被光和酶激活的基团封闭的能力,因此激活策略是高度模块化的。这项工作描述了具有新核心支架的下一代可激活环丙烯,该支架保持了第一代的激活模块性,但与s-四嗪的连接动力学提高了≤270倍。
更新日期:2019-04-24
中文翻译:

笼型环丙烯具有改善的四嗪连接反应动力学
可活化的环丙烯对它们的逆电子需求Diels–Alder反应伙伴(例如,s-四嗪)不发生反应,直到被活化为止。由于环丙烯具有被各种可被光和酶激活的基团封闭的能力,因此激活策略是高度模块化的。这项工作描述了具有新核心支架的下一代可激活环丙烯,该支架保持了第一代的激活模块性,但与s-四嗪的连接动力学提高了≤270倍。































 京公网安备 11010802027423号
京公网安备 11010802027423号