当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anti-EpCAM-conjugated adeno-associated virus serotype 2 for systemic delivery of EGFR shRNA: Its retargeting and antitumor effects on OVCAR3 ovarian cancer in vivo.
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-04-23 , DOI: 10.1016/j.actbio.2019.04.044 Sungjin Lee 1 , Hyung Jun Ahn 1
Acta Biomaterialia ( IF 9.4 ) Pub Date : 2019-04-23 , DOI: 10.1016/j.actbio.2019.04.044 Sungjin Lee 1 , Hyung Jun Ahn 1
Affiliation
|
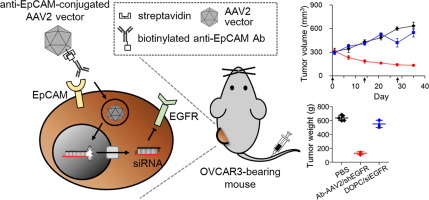
Adeno-associated virus (AAV) is a promising vector for systemic delivery of siRNA because of its long-term expression ability without immunogenicity and pathogenicity. However, its broad host tropism and lack of tissue specificity have limited clinical applications such as cancer therapy. Therefore, redirecting the natural tropism of AAV vectors to unique cell surface antigens is an important requirement for in vivo RNAi-based cancer therapy. To use the overexpression property of epithelial cell adhesion molecule (EpCAM) in specific cancer types, we herein created anti-EpCAM antibody-conjugated AAV serotype 2 (AAV2) vectors through a streptavidin-biotin bridge. Upon intravenous injection, anti-EpCAM-conjugated AAV2 vectors showed prominent tumor-specific accumulation in EpCAM-positive tumor-bearing mice without undesirable sequestration in liver. In addition, when loaded with transgenes to express shRNA against epidermal growth factor receptor (EGFR), systemically injected anti-EpCAM-conjugated AAV2/shEGFR vectors induced significant downregulation of EGFR expression in tumors and eventually suppressed tumor growth even at the long dosing interval of two weeks. This in vivo antitumor effect represents the increased infection efficacy of tropism-modified AAV2 vectors and prolonged expression of EGFR shRNA in tumor tissues. Thus, this study suggests the great potential of anti-EpCAM-conjugated AAV2/shEGFR vectors as RNAi-based cancer therapeutics. STATEMENT OF SIGNIFICANCE: Adeno-associated virus (AAV) is a promising vector for systemic delivery of siRNA, but its broad host tropism has limited clinical applications. By using the overexpression property of epithelial cell adhesion molecule (EpCAM) on tumors, we demonstrate that anti-EpCAM-conjugated AAV2 vectors through a streptavidin-biotin bridge are redirected to EpCAM-positive tumors in vivo. In addition, when loaded with transgenes to express shRNA against epidermal growth factor receptor (EGFR), systemically injected anti-EpCAM-conjugated AAV2/shEGFR vectors significantly downregulate EGFR expression in tumors, eventually suppressing tumor growth for long periods. We herein suggest the potential of anti-EpCAM-AAV2/shEGFR vectors as an antitumor agent. Furthermore, redirection of AAV2 infection through EpCAM would provide a powerful means for systemic delivery of short hairpin RNA to tumor sites.
中文翻译:

用于全身递送EGFR shRNA的抗EpCAM缀合的腺相关病毒血清型2:在体内对OVCAR3卵巢癌的重靶向和抗肿瘤作用。
腺伴随病毒(AAV)是一种有希望的系统递送siRNA的载体,因为它的长期表达能力没有免疫原性和致病性。然而,其广泛的宿主嗜性和组织特异性的缺乏限制了诸如癌症治疗之类的临床应用。因此,将AAV载体的天然定向性重定向到独特的细胞表面抗原是基于体内RNAi的癌症治疗的重要要求。为了在特定的癌症类型中使用上皮细胞粘附分子(EpCAM)的过表达特性,我们在这里通过链霉亲和素-生物素桥创建了抗EpCAM抗体偶联的AAV血清型2(AAV2)载体。静脉注射后,抗EpCAM缀合的AAV2载体在EpCAM阳性荷瘤小鼠中显示出明显的肿瘤特异性蓄积,而在肝脏中没有不希望的隔离。此外,当装载转基因以表达针对表皮生长因子受体(EGFR)的shRNA时,系统注射的抗EpCAM偶联的AAV2 / shEGFR载体可诱导肿瘤中EGFR表达的显着下调,甚至在长剂量给药间隔下也可抑制肿瘤生长。两个星期。这种体内抗肿瘤作用代表了向性修饰的AAV2载体提高的感染功效和肿瘤组织中EGFR shRNA的延长表达。因此,这项研究表明抗EpCAM偶联的AAV2 / shEGFR载体作为基于RNAi的癌症治疗剂的巨大潜力。意义声明:腺伴随病毒(AAV)是用于siRNA系统递送的有前途的载体,但其广泛的宿主嗜性限制了其临床应用。通过使用上皮细胞粘附分子(EpCAM)在肿瘤上的过表达特性,我们证明抗EpCAM缀合的AAV2载体通过链霉亲和素-生物素桥被重定向到体内的EpCAM阳性肿瘤。另外,当装载转基因以表达针对表皮生长因子受体(EGFR)的shRNA时,系统注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。我们证明通过抗生蛋白链菌素-生物素桥的抗EpCAM缀合的AAV2载体在体内重定向到EpCAM阳性肿瘤。另外,当装载转基因以表达针对表皮生长因子受体(EGFR)的shRNA时,系统注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。我们证明通过抗生蛋白链菌素-生物素桥的抗EpCAM缀合的AAV2载体在体内重定向到EpCAM阳性肿瘤。另外,当装载转基因以表达针对表皮生长因子受体(EGFR)的shRNA时,系统注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。全身注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。全身注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。
更新日期:2019-05-16
中文翻译:

用于全身递送EGFR shRNA的抗EpCAM缀合的腺相关病毒血清型2:在体内对OVCAR3卵巢癌的重靶向和抗肿瘤作用。
腺伴随病毒(AAV)是一种有希望的系统递送siRNA的载体,因为它的长期表达能力没有免疫原性和致病性。然而,其广泛的宿主嗜性和组织特异性的缺乏限制了诸如癌症治疗之类的临床应用。因此,将AAV载体的天然定向性重定向到独特的细胞表面抗原是基于体内RNAi的癌症治疗的重要要求。为了在特定的癌症类型中使用上皮细胞粘附分子(EpCAM)的过表达特性,我们在这里通过链霉亲和素-生物素桥创建了抗EpCAM抗体偶联的AAV血清型2(AAV2)载体。静脉注射后,抗EpCAM缀合的AAV2载体在EpCAM阳性荷瘤小鼠中显示出明显的肿瘤特异性蓄积,而在肝脏中没有不希望的隔离。此外,当装载转基因以表达针对表皮生长因子受体(EGFR)的shRNA时,系统注射的抗EpCAM偶联的AAV2 / shEGFR载体可诱导肿瘤中EGFR表达的显着下调,甚至在长剂量给药间隔下也可抑制肿瘤生长。两个星期。这种体内抗肿瘤作用代表了向性修饰的AAV2载体提高的感染功效和肿瘤组织中EGFR shRNA的延长表达。因此,这项研究表明抗EpCAM偶联的AAV2 / shEGFR载体作为基于RNAi的癌症治疗剂的巨大潜力。意义声明:腺伴随病毒(AAV)是用于siRNA系统递送的有前途的载体,但其广泛的宿主嗜性限制了其临床应用。通过使用上皮细胞粘附分子(EpCAM)在肿瘤上的过表达特性,我们证明抗EpCAM缀合的AAV2载体通过链霉亲和素-生物素桥被重定向到体内的EpCAM阳性肿瘤。另外,当装载转基因以表达针对表皮生长因子受体(EGFR)的shRNA时,系统注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。我们证明通过抗生蛋白链菌素-生物素桥的抗EpCAM缀合的AAV2载体在体内重定向到EpCAM阳性肿瘤。另外,当装载转基因以表达针对表皮生长因子受体(EGFR)的shRNA时,系统注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。我们证明通过抗生蛋白链菌素-生物素桥的抗EpCAM缀合的AAV2载体在体内重定向到EpCAM阳性肿瘤。另外,当装载转基因以表达针对表皮生长因子受体(EGFR)的shRNA时,系统注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。全身注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。全身注射的抗EpCAM偶联的AAV2 / shEGFR载体显着下调了肿瘤中EGFR的表达,最终长期抑制了肿瘤的生长。我们在本文中提出了抗EpCAM-AAV2 / shEGFR载体作为抗肿瘤剂的潜力。此外,通过EpCAM重定向AAV2感染将为将短发夹RNA全身性递送至肿瘤部位提供强有力的手段。















































 京公网安备 11010802027423号
京公网安备 11010802027423号