当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Specific Targeting, Imaging, and Ablation of Tumor-Associated Macrophages by Theranostic Mannose-AIEgen Conjugates.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-04-22 00:00:00 , DOI: 10.1021/acs.analchem.9b01053 Xiaoying Gao 1, 2 , Duo Mao 2 , Xingang Zuo 1 , Fang Hu 2 , Jie Cao 1 , Peng Zhang 1 , Jing Zhi Sun 1 , Jianzhao Liu 1 , Bin Liu 2 , Ben Zhong Tang 1, 3, 4
Analytical Chemistry ( IF 6.7 ) Pub Date : 2019-04-22 00:00:00 , DOI: 10.1021/acs.analchem.9b01053 Xiaoying Gao 1, 2 , Duo Mao 2 , Xingang Zuo 1 , Fang Hu 2 , Jie Cao 1 , Peng Zhang 1 , Jing Zhi Sun 1 , Jianzhao Liu 1 , Bin Liu 2 , Ben Zhong Tang 1, 3, 4
Affiliation
|
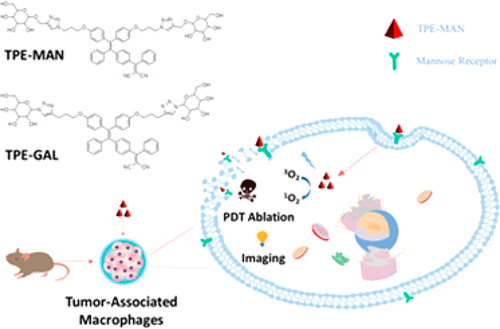
Tumor-associated macrophages (TAMs) that exist in tumor microenvironment promote tumor progression and have been suggested as a promising therapeutic target for cancer therapy in preclinical studies. Development of theranostic systems capable of specific targeting, imaging, and ablation of TAMs will offer clinical benefits. Here we constructed a theranostic probe, namely, TPE-Man, by attaching mannose moieties to a red-emissive and AIE (aggregation-induced emission)-active photosensitizer. TPE-Man can specifically recognize a mannose receptor that is overexpressed on TAMs by the sugar–receptor interaction and enables fluorescent visualization of the mannose-receptor-positive TAMs in high contrast. The histologic study of mouse tumor sections further verifies TPE-Man’s excellent targeting specificity being comparable with the commercial mannose-receptor antibody. TAMs can be effectively eradicated upon exposure to white light irradiation via a photodynamic therapy effect. To our knowledge, this is the first small molecular theranostic probe for TAMs that revealed combined advantages of low cost, high targeting specificity, fluorescent light-up imaging, and efficient photodynamic ablation.
中文翻译:

肿瘤相关的巨噬细胞的特定靶向,成像和消融的治疗性甘露糖-AIEgen结合。
存在于肿瘤微环境中的肿瘤相关巨噬细胞(TAM)促进肿瘤进展,并且在临床前研究中已被建议作为癌症治疗的有希望的治疗靶标。能够特异性靶向,成像和消融TAM的治疗治疗系统将提供临床益处。在这里,我们通过将甘露糖部分连接到红色发射和AIE(聚集诱导发射)活性光敏剂上,构建了一种治疗学探针,即TPE-Man。TPE-Man可以特异性识别通过糖-受体相互作用在TAM上过表达的甘露糖受体,并能以高对比度荧光显示甘露糖-受体阳性TAM。小鼠肿瘤切片的组织学研究进一步证实了TPE-Man的出色靶向特异性可与市售甘露糖受体抗体相媲美。通过光动力疗法作用,在暴露于白光照射下可以有效地消除TAM。据我们所知,这是第一款用于TAM的小分子治疗诊断探针,显示出低成本,高靶向特异性,荧光灯成像和有效的光动力消融相结合的优势。
更新日期:2019-04-22
中文翻译:

肿瘤相关的巨噬细胞的特定靶向,成像和消融的治疗性甘露糖-AIEgen结合。
存在于肿瘤微环境中的肿瘤相关巨噬细胞(TAM)促进肿瘤进展,并且在临床前研究中已被建议作为癌症治疗的有希望的治疗靶标。能够特异性靶向,成像和消融TAM的治疗治疗系统将提供临床益处。在这里,我们通过将甘露糖部分连接到红色发射和AIE(聚集诱导发射)活性光敏剂上,构建了一种治疗学探针,即TPE-Man。TPE-Man可以特异性识别通过糖-受体相互作用在TAM上过表达的甘露糖受体,并能以高对比度荧光显示甘露糖-受体阳性TAM。小鼠肿瘤切片的组织学研究进一步证实了TPE-Man的出色靶向特异性可与市售甘露糖受体抗体相媲美。通过光动力疗法作用,在暴露于白光照射下可以有效地消除TAM。据我们所知,这是第一款用于TAM的小分子治疗诊断探针,显示出低成本,高靶向特异性,荧光灯成像和有效的光动力消融相结合的优势。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号