Tetrahedron ( IF 2.1 ) Pub Date : 2019-04-20 , DOI: 10.1016/j.tet.2019.04.019 Jiaming Liu , Shang Gao , Ming Chen
|
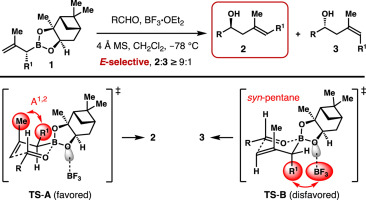
Enantioselective allylboration of aldehydes with α-substituted β-methyl allylboronate was reported. By using BF3·OEt2 as the catalyst, γ,δ-disubstituted homoallylic alcohols were obtained in good yields with high E-selectivities and enantioselectivities. Transition state analysis revealed that the disfavored transition state suffers from a syn-pentane interaction between the BF3 catalyst and axially oriented α-substituent of the allylboron reagent. Such a syn-pentane interaction is severe enough to overcome the A1,2 allylic strain between the β-methyl group and the α-substituent of the boron reagent that is present in the favored competing transition state. Consequently, the reaction proceeded with equatorial placement of the α-substituent to furnish γ-methyl substituted homoallylic alcohols with high E-selectivity.
中文翻译:

(对映选择性合成ë)-γ,δ二取代经由BF高烯丙醇3 ·OET 2 -催化的醛allylboration和起源的分析Ë一个: -选择性1,2-烯丙基应变与顺式-戊烷相互作用
报道了醛与α-取代的β-甲基烯丙基硼酸酯的对映选择性烯丙基硼化。以BF 3 ·OEt 2为催化剂,可以得到高产率,高E选择性和对映选择性的γ,δ-二取代均烯丙基醇。过渡状态分析表明,从该不受欢迎过渡状态患有顺式的BF之间戊烷相互作用3催化剂和allylboron试剂的轴向定向的α取代基。这样的顺式-戊烷相互作用是严重足以克服甲1,2-以有利的竞争过渡态存在的硼试剂的β-甲基和α-取代基之间的烯丙基应变。因此,该反应在赤道放置α-取代基的情况下进行,以提供具有高E-选择性的γ-甲基取代的均烯丙基醇。































 京公网安备 11010802027423号
京公网安备 11010802027423号