当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of H3+ through Chloromethane Dication Fragmentation
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-04-18 00:00:00 , DOI: 10.1021/acsearthspacechem.9b00045 Jérôme Palaudoux 1 , Majdi Hochlaf 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2019-04-18 00:00:00 , DOI: 10.1021/acsearthspacechem.9b00045 Jérôme Palaudoux 1 , Majdi Hochlaf 2
Affiliation
|
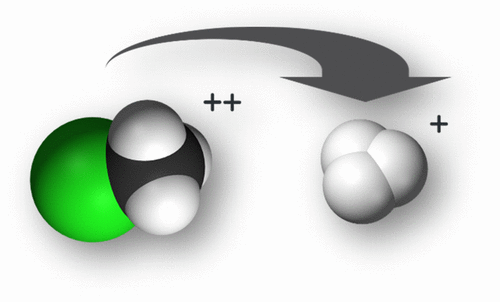
Using post Hartree–Fock Multi Reference Configuration Interaction methods, we performed suitable one-dimensional cuts of the potential energy surfaces of the lowest electronic states of the CH3Cl2+ dication. Calculations show that the CH3Cl2+ dication fragments efficiently produce the astrophysical, planetary, and atmospherically important H3+ cation. A new multistep mechanism is proposed, where the three hydrogens of this methyl halide approach each other simultaneously to form H3+ in conjunction with singlet–triplet spin–orbit and internal conversions. The CH3Cl2+ dication can be formed after single or multiple ionizations of the already detected CH3Cl neutral molecule. Our results may be used for the interpretation of the formation of H3+ in laboratory and in astrophysical media.
中文翻译:

通过氯甲烷阳离子裂解形成H 3 +
使用后Hartree-Fock多参考配置相互作用方法,我们对CH 3 Cl 2+指示剂的最低电子态的势能面进行了适当的一维切割。计算表明,CH 3 Cl 2+离子碎片有效地产生了天体,行星和大气上重要的H 3 +阳离子。提出了一种新的多步机制,其中该甲基卤化物的三个氢原子同时接近并形成单重态-三重态自旋轨道和内部转化,形成H 3 +。CH 3 Cl 2+可以在已经检测到的CH 3 Cl中性分子单次或多次电离后形成离子。我们的结果可用于解释实验室和天体物理介质中H 3 +的形成。
更新日期:2019-04-18
中文翻译:

通过氯甲烷阳离子裂解形成H 3 +
使用后Hartree-Fock多参考配置相互作用方法,我们对CH 3 Cl 2+指示剂的最低电子态的势能面进行了适当的一维切割。计算表明,CH 3 Cl 2+离子碎片有效地产生了天体,行星和大气上重要的H 3 +阳离子。提出了一种新的多步机制,其中该甲基卤化物的三个氢原子同时接近并形成单重态-三重态自旋轨道和内部转化,形成H 3 +。CH 3 Cl 2+可以在已经检测到的CH 3 Cl中性分子单次或多次电离后形成离子。我们的结果可用于解释实验室和天体物理介质中H 3 +的形成。































 京公网安备 11010802027423号
京公网安备 11010802027423号