当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis and photoinduced DNA cleavage studies of [1,2,4]-triazolo[4,3-a]quinoxalin-4(5H)-ones.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-04-19 , DOI: 10.1016/j.bioorg.2019.102932 Garima Sumran 1 , Ranjana Aggarwal 2 , Ashwani Mittal 3 , Aviral Aggarwal 4 , Amit Gupta 5
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-04-19 , DOI: 10.1016/j.bioorg.2019.102932 Garima Sumran 1 , Ranjana Aggarwal 2 , Ashwani Mittal 3 , Aviral Aggarwal 4 , Amit Gupta 5
Affiliation
|
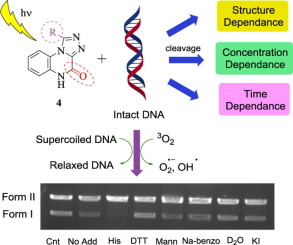
An expedient and eco-friendly synthesis of 1-aryl/heteroaryl-[1,2,4]-triazolo[4,3-a]quinoxalin-4(5H)-ones (4) has been accomplished via iodobenzene diacetate mediated oxidative intramolecular cyclization of 3-(2-(aryl/heteroarylidene)hydrazinyl)-quinoxalin-2(1H)-ones (3). Ten synthesized compounds 3 and 4 (10-40 μg) on irradiation with UV light at λmax 312 nm could lead to cleavage of supercoiled pMaxGFP DNA (Form I) into the relaxed DNA (Form II) without any additive. Further, DNA cleaving ability of triazoles was quantitatively evaluated and was found to be dependent on its structure, concentration, and strictly on photoirradiation time. Mechanistic investigations using several additives as potential inhibitors/activator revealed that the DNA photocleavage reaction involves Type-I pathway leading to formation of superoxide anion radicals (O2-) as the major reactive oxygen species responsible for photocleavage process.
中文翻译:

[1,2,4]-三唑并[4,3-a]喹喔啉-4(5H)-ones的设计,合成和光诱导的DNA裂解研究。
通过碘代苯二乙酸酯介导的氧化性分子内合成1-芳基/杂芳基-[1,2,4]-三唑并[4,3-a]喹喔啉-4(5H)-ones(4)的简便方法3-(2-(芳基/杂芳基)肼基)-喹喔啉-2(1H)-1的环化(3)。十个合成的化合物3和4(10-40μg)在λmax312 nm的紫外线照射下可能导致超螺旋pMaxGFP DNA(I型)裂解为松弛的DNA(II型),而没有任何添加剂。此外,对三唑的DNA切割能力进行了定量评估,发现其结构,浓度和严格取决于光辐照时间。
更新日期:2019-04-19
中文翻译:

[1,2,4]-三唑并[4,3-a]喹喔啉-4(5H)-ones的设计,合成和光诱导的DNA裂解研究。
通过碘代苯二乙酸酯介导的氧化性分子内合成1-芳基/杂芳基-[1,2,4]-三唑并[4,3-a]喹喔啉-4(5H)-ones(4)的简便方法3-(2-(芳基/杂芳基)肼基)-喹喔啉-2(1H)-1的环化(3)。十个合成的化合物3和4(10-40μg)在λmax312 nm的紫外线照射下可能导致超螺旋pMaxGFP DNA(I型)裂解为松弛的DNA(II型),而没有任何添加剂。此外,对三唑的DNA切割能力进行了定量评估,发现其结构,浓度和严格取决于光辐照时间。































 京公网安备 11010802027423号
京公网安备 11010802027423号