当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved Electrocatalytic Water Splitting Reaction on CeO2(111) by Strain Engineering: A DFT+U Study
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-04-18 00:00:00 , DOI: 10.1021/acscatal.9b00203 Tiantian Wu 1 , Tejs Vegge 1 , Heine Anton Hansen 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-04-18 00:00:00 , DOI: 10.1021/acscatal.9b00203 Tiantian Wu 1 , Tejs Vegge 1 , Heine Anton Hansen 1
Affiliation
|
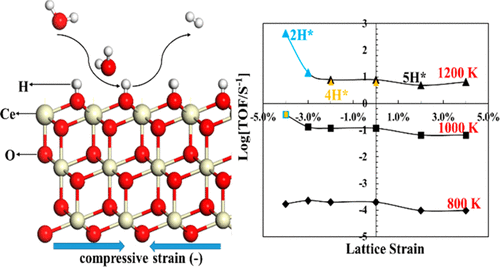
Ceria is a promising cathode material in solid oxide electrolysis cells (SOECs) because ceria can become a mixed electronic and ionic conductor through doping, which enables a high surface area for electrocatalysis. Here, we systemically investigate the effect of strain on the electrocatalytic water splitting reaction (WSR) for renewable hydrogen production on CeO2(111) by using density functional theory corrected for on-site Coulomb interactions (DFT+U). We find that tensile strain stabilizes the reduced states of ceria such as oxygen vacancies and surface hydroxyls, while compressive strain destabilizes the reduced states. These trends are explained by a downshift of the Ce 4f orbital energy under tensile strain and agree with the larger size of the Ce3+ ion in comparison to the Ce4+ ion. Our results show that hydroxyl decomposition into H2 has the highest activation energy along the WSR pathway (Ea) and that the free energy of hydroxyl formation (ΔGH) prior to hydroxyl decomposition can act as a thermodynamic barrier to the WSR. Compressive strain (<−3.0%) correlates strongly with increased WSR activity on CeO2(111) because it reduces the total barrier (ΔGH + Ea). Strain also effectively engineers the reaction pathway of the WSR at T > 1000 K. By a comparison of the total reaction barrier on different hydroxylated surfaces, the WSR is found to proceed readily via a Ce–H intermediate on excessively hydroxylated CeO2(111) under tensile strain because of the lower barrier, while the WSR proceeds preferentially and readily on the partially or fully hydroxylated CeO2(111) under compressive strain. In addition, a direct mapping between the most efficient WSR pathway and strain at different operating temperatures provides a better understanding of the efficient WSR on the CeO2(111) facet by strain engineering, which sheds light on electrocatalysis on oxide catalysts.
中文翻译:

通过应变工程改进的CeO 2(111)电催化水分解反应:DFT + U研究
二氧化铈是固体氧化物电解槽(SOEC)中很有希望的阴极材料,因为二氧化铈可以通过掺杂变成混合的电子和离子导体,从而可以实现高表面积的电催化作用。在这里,我们通过使用针对现场库仑相互作用(DFT + U)校正的密度泛函理论,系统地研究了应变对CeO 2(111)上可再生氢生产的电催化水分解反应(WSR)的影响。我们发现,拉伸应变可以稳定二氧化铈的还原态,例如氧空位和表面羟基,而压缩应变可以使还原态不稳定。这些趋势可以通过拉伸应变作用下Ce 4f轨道能量的下移来解释,并且与Ce 3+的较大尺寸一致与Ce 4+离子相比。我们的结果表明,羟基分解成H 2沿WSR途径具有最高的活化能(E a),羟基分解之前羟基形成的自由能(ΔG H)可以作为WSR的热力学屏障。压缩应变(<-3.0%)与CeO 2(111)的WSR活性增加密切相关,因为它降低了总势垒(ΔG H + E a)。应变还有效地设计了WSR在T处的反应途径> 1000K。通过比较不同羟基化表面上的总反应势垒,发现WSR易于通过Ce-H中间体在张力应变下通过过度羟基化的CeO 2(111)上的Ce-H中间体进行,因为该势垒较低,而WSR在压缩应变下,在部分或完全羟基化的CeO 2(111)上优先且容易地进行反应。此外,在不同的工作温度下,最有效的WSR路径与应变之间的直接映射关系,通过应变工程可以更好地理解CeO 2(111)面上的有效WSR ,从而为氧化物催化剂的电催化提供了亮点。
更新日期:2019-04-18
中文翻译:

通过应变工程改进的CeO 2(111)电催化水分解反应:DFT + U研究
二氧化铈是固体氧化物电解槽(SOEC)中很有希望的阴极材料,因为二氧化铈可以通过掺杂变成混合的电子和离子导体,从而可以实现高表面积的电催化作用。在这里,我们通过使用针对现场库仑相互作用(DFT + U)校正的密度泛函理论,系统地研究了应变对CeO 2(111)上可再生氢生产的电催化水分解反应(WSR)的影响。我们发现,拉伸应变可以稳定二氧化铈的还原态,例如氧空位和表面羟基,而压缩应变可以使还原态不稳定。这些趋势可以通过拉伸应变作用下Ce 4f轨道能量的下移来解释,并且与Ce 3+的较大尺寸一致与Ce 4+离子相比。我们的结果表明,羟基分解成H 2沿WSR途径具有最高的活化能(E a),羟基分解之前羟基形成的自由能(ΔG H)可以作为WSR的热力学屏障。压缩应变(<-3.0%)与CeO 2(111)的WSR活性增加密切相关,因为它降低了总势垒(ΔG H + E a)。应变还有效地设计了WSR在T处的反应途径> 1000K。通过比较不同羟基化表面上的总反应势垒,发现WSR易于通过Ce-H中间体在张力应变下通过过度羟基化的CeO 2(111)上的Ce-H中间体进行,因为该势垒较低,而WSR在压缩应变下,在部分或完全羟基化的CeO 2(111)上优先且容易地进行反应。此外,在不同的工作温度下,最有效的WSR路径与应变之间的直接映射关系,通过应变工程可以更好地理解CeO 2(111)面上的有效WSR ,从而为氧化物催化剂的电催化提供了亮点。













































 京公网安备 11010802027423号
京公网安备 11010802027423号