当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanistic Insights into the Directing Effect of Thr303 in Ethanol Oxidation by Cytochrome P450 2E1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-04-18 00:00:00 , DOI: 10.1021/acscatal.9b00907 Qianqian Lu 1, 2 , Jinshuai Song 1, 2 , Peng Wu 1, 3 , Chunsen Li 1, 2 , Walter Thiel 4
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-04-18 00:00:00 , DOI: 10.1021/acscatal.9b00907 Qianqian Lu 1, 2 , Jinshuai Song 1, 2 , Peng Wu 1, 3 , Chunsen Li 1, 2 , Walter Thiel 4
Affiliation
|
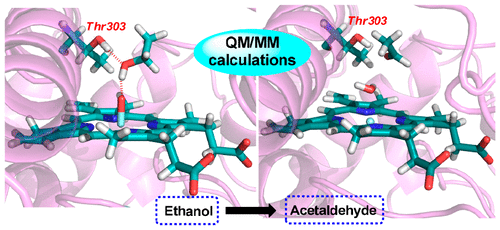
There is a long-standing mechanistic consensus that alcohol oxidation by cytochrome P450 enzymes is triggered by hydrogen abstraction from the α-C–H bond of the alcohol. Through combined molecular dynamics simulations and quantum mechanics/molecular mechanics calculations we demonstrate that this is not the case in P450 2E1-mediated ethanol oxidation. We show that while the O–H bond is stronger than the α-C–H bonds in alcohols, the intrinsic reactivity of O–H and α-C–H bonds is comparable for hydrogen abstraction, due to the strong electrostatic interaction between the ethanol hydroxyl group and the Fe═O moiety. Thus, the binding of ethanol to the Fe═O moiety in the P450 2E1 pocket is of particular importance to the reaction mechanism. We further show that the Thr303 residue plays a crucial role in confining the ethanol substrate in the active site of P450 2E1 and thereby steering the initial hydrogen abstraction from the O–H bond of ethanol. Because of the highly endothermic O–H bond cleavage, the subsequent hydrogen abstraction of α-C–H bond is the overall rate-determining step for ethanol oxidation. These mechanistic findings are in agreement with available experimental data (e.g., kinetic isotope experiments and electron spin resonance analysis). Our work sheds light on the puzzling mechanism of ethanol oxidation in P450 2E1 by identifying the directing effect of Thr303 on substrate orientation, which complements its role as a proton-shuttle mediator during the formation of Compound I.
中文翻译:

机械洞察Thr303指导细胞色素P450 2E1氧化乙醇的作用。
长期以来,在机理上已经达成共识,即细胞色素P450酶的醇氧化是由醇的α-C-H键夺氢引起的。通过结合分子动力学模拟和量子力学/分子力学计算,我们证明在P450 2E1介导的乙醇氧化中并非如此。我们表明,虽然醇中的O–H键比α-CH–H键更强,但由于氢之间的强静电相互作用,OH和α-CH–H键的固有反应性可与氢的提取相媲美。乙醇羟基和Fe═O部分。因此,乙醇与P450 2E1口袋中Fe 3 O部分的结合对反应机理特别重要。我们进一步表明,Thr303残基在限制P450 2E1的活性位点中的乙醇底物,从而控制乙醇的O–H键的初始氢提取中起着至关重要的作用。由于高度吸热的O–H键裂解,随后的α-CH–H键氢抽象是乙醇氧化的总体速率决定步骤。这些机制的发现与可用的实验数据(例如,动力学同位素实验和电子自旋共振分析)一致。我们的工作通过确定Thr303对底物取向的指导作用来阐明P450 2E1中乙醇氧化的令人困惑的机理,这补充了它在化合物I形成过程中作为质子-穿梭介质的作用。
更新日期:2019-04-18
中文翻译:

机械洞察Thr303指导细胞色素P450 2E1氧化乙醇的作用。
长期以来,在机理上已经达成共识,即细胞色素P450酶的醇氧化是由醇的α-C-H键夺氢引起的。通过结合分子动力学模拟和量子力学/分子力学计算,我们证明在P450 2E1介导的乙醇氧化中并非如此。我们表明,虽然醇中的O–H键比α-CH–H键更强,但由于氢之间的强静电相互作用,OH和α-CH–H键的固有反应性可与氢的提取相媲美。乙醇羟基和Fe═O部分。因此,乙醇与P450 2E1口袋中Fe 3 O部分的结合对反应机理特别重要。我们进一步表明,Thr303残基在限制P450 2E1的活性位点中的乙醇底物,从而控制乙醇的O–H键的初始氢提取中起着至关重要的作用。由于高度吸热的O–H键裂解,随后的α-CH–H键氢抽象是乙醇氧化的总体速率决定步骤。这些机制的发现与可用的实验数据(例如,动力学同位素实验和电子自旋共振分析)一致。我们的工作通过确定Thr303对底物取向的指导作用来阐明P450 2E1中乙醇氧化的令人困惑的机理,这补充了它在化合物I形成过程中作为质子-穿梭介质的作用。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号