当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and molecular docking study of some 3,4-dihydrothieno[2,3-d]pyrimidine derivatives as potential antimicrobial agents.
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-04-16 , DOI: 10.1016/j.bioorg.2019.102934 Omaima G Shaaban 1 , Doaa A E Issa 2 , Alaa A El-Tombary 3 , Shrouk M Abd El Wahab 4 , Abeer E Abdel Wahab 5 , Ibrahim A Abdelwahab 6
Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-04-16 , DOI: 10.1016/j.bioorg.2019.102934 Omaima G Shaaban 1 , Doaa A E Issa 2 , Alaa A El-Tombary 3 , Shrouk M Abd El Wahab 4 , Abeer E Abdel Wahab 5 , Ibrahim A Abdelwahab 6
Affiliation
|
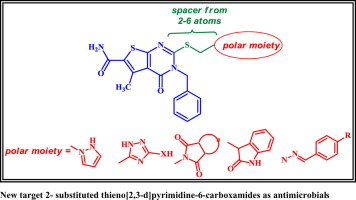
In continuation of our research program aiming at developing new potent antimicrobial agents, new series of substituted 3,4-dihydrothieno[2,3-d]pyrimidines was synthesized. The newly synthesized compounds were preliminary tested for their in vitro activity against six bacterial and three fungal strains using the agar diffusion technique. The results revealed that compounds 7, 8a, 10b, 10d and 11b exhibited half the potency of levofloxacine against the Gram-negative bacterium, Pseudomonas aeruginosa, while compounds 5a, 8b, 10c and 12 displayed half the potency of levofloxacine against Proteus Vulgaris. Whereas, compounds 7, 10b, 10d and 11b showed half the activity of ampicillin against the Gram-positive bacterium, B. subtilis. Most of the compounds showed high antifungal potency. Compounds 3, 6, 7, 9b, 10a, 11a, 11b, 15 and 16 exhibited double the potency of clotrimazole against A. fumigatus. While compounds 3, 4, 5a, 5b, 9b, 10a, 10b, 10c, 13, 15, 16 and 18 displayed double the activity of clotrimazole against R. oryazae. Molecular docking studies of the active compounds with the active site of the B. anthracis DHPS, showed good scoring for various interactions with the active site of the enzyme compared to the co-crystallized ligand.
中文翻译:

一些3,4-二氢噻吩并[2,3-d]嘧啶衍生物的合成和分子对接研究作为潜在的抗菌剂。
在继续致力于开发新型有效抗菌剂的研究计划的过程中,合成了一系列新的取代的3,4-二氢噻吩并[2,3-d]嘧啶类化合物。使用琼脂扩散技术,初步测试了新合成的化合物对六种细菌和三种真菌菌株的体外活性。结果表明,化合物7、8a,10b,10d和11b表现出左氧氟沙星对革兰氏阴性细菌铜绿假单胞菌的效力的一半,而化合物5a,8b,10c和12表现出左氧氟沙星对变形杆菌的效力的一半。而化合物7、10b,10d和11b则显示出氨苄西林对革兰氏阳性细菌枯草芽孢杆菌的一半活性。大多数化合物显示出很高的抗真菌效力。化合物3、6、7、9b,10a,11a,11b,图15和16显示出克霉唑对烟曲霉的效力是其两倍。化合物3、4、5a,5b,9b,10a,10b,10c,13、15、16和18显示出克霉唑对米根霉的活性翻倍。活性化合物与炭疽杆菌DHPS活性位点的分子对接研究显示,与共结晶的配体相比,与酶活性位点的各种相互作用均获得了良好的评分。
更新日期:2019-04-16
中文翻译:

一些3,4-二氢噻吩并[2,3-d]嘧啶衍生物的合成和分子对接研究作为潜在的抗菌剂。
在继续致力于开发新型有效抗菌剂的研究计划的过程中,合成了一系列新的取代的3,4-二氢噻吩并[2,3-d]嘧啶类化合物。使用琼脂扩散技术,初步测试了新合成的化合物对六种细菌和三种真菌菌株的体外活性。结果表明,化合物7、8a,10b,10d和11b表现出左氧氟沙星对革兰氏阴性细菌铜绿假单胞菌的效力的一半,而化合物5a,8b,10c和12表现出左氧氟沙星对变形杆菌的效力的一半。而化合物7、10b,10d和11b则显示出氨苄西林对革兰氏阳性细菌枯草芽孢杆菌的一半活性。大多数化合物显示出很高的抗真菌效力。化合物3、6、7、9b,10a,11a,11b,图15和16显示出克霉唑对烟曲霉的效力是其两倍。化合物3、4、5a,5b,9b,10a,10b,10c,13、15、16和18显示出克霉唑对米根霉的活性翻倍。活性化合物与炭疽杆菌DHPS活性位点的分子对接研究显示,与共结晶的配体相比,与酶活性位点的各种相互作用均获得了良好的评分。








































 京公网安备 11010802027423号
京公网安备 11010802027423号