当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
2-Methoxyphenyl isocyanate: a chemoselective multitasking reagent for an amine protection/deprotection sequence†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019-04-17 00:00:00 , DOI: 10.1039/c9qo00293f Anand Babu Velappan 1, 2, 3, 4 , Subhashini Kogatam 1, 2, 3, 4 , Dhrubajyoti Datta 1, 4, 5 , Rakshantha Srithar 1, 2, 3, 4 , Gunasekaran Nanjappan 2, 3, 4, 6 , Joy Debnath 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2019-04-17 00:00:00 , DOI: 10.1039/c9qo00293f Anand Babu Velappan 1, 2, 3, 4 , Subhashini Kogatam 1, 2, 3, 4 , Dhrubajyoti Datta 1, 4, 5 , Rakshantha Srithar 1, 2, 3, 4 , Gunasekaran Nanjappan 2, 3, 4, 6 , Joy Debnath 1, 2, 3, 4
Affiliation
|
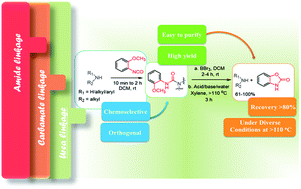
Organic amines, in general, are protected through carbamate bond mediated cappings. Herein, we have demonstrated the chemically stable urea linkage suitably employed for protection/deptrotection of amino groups. The stability of the urea linkage under acidic, alkaline and aqueous conditions is an additional advantage for such a protecting group. Therefore the chemoselective nature of 2-methoxyphenyl isocyanate enables its use as a new class of protecting groups which can regenerate free amines after a convenient deprotection step.
中文翻译:

2-甲氧基苯基异氰酸酯:用于胺保护/脱保护序列的化学选择性多任务试剂†
通常,有机胺通过氨基甲酸酯键介导的封端保护。在本文中,我们已经证明了化学上稳定的脲键,其适合用于氨基的保护/二肽保护。脲键在酸性,碱性和水性条件下的稳定性是这种保护基团的另一个优点。因此,2-甲氧基苯基异氰酸酯的化学选择性性质使其可以用作一类新的保护基团,其可以在方便的脱保护步骤后再生游离胺。
更新日期:2019-04-17
中文翻译:

2-甲氧基苯基异氰酸酯:用于胺保护/脱保护序列的化学选择性多任务试剂†
通常,有机胺通过氨基甲酸酯键介导的封端保护。在本文中,我们已经证明了化学上稳定的脲键,其适合用于氨基的保护/二肽保护。脲键在酸性,碱性和水性条件下的稳定性是这种保护基团的另一个优点。因此,2-甲氧基苯基异氰酸酯的化学选择性性质使其可以用作一类新的保护基团,其可以在方便的脱保护步骤后再生游离胺。






























 京公网安备 11010802027423号
京公网安备 11010802027423号