当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identification of Filamin A Mechanobinding Partner II: Fimbacin Is a Novel Actin Cross-Linking and Filamin A Binding Protein.
Biochemistry ( IF 2.9 ) Pub Date : 2019-04-24 , DOI: 10.1021/acs.biochem.9b00101
Jiale Wang 1 , Fumihiko Nakamura 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-04-24 , DOI: 10.1021/acs.biochem.9b00101
Jiale Wang 1 , Fumihiko Nakamura 1
Affiliation
|
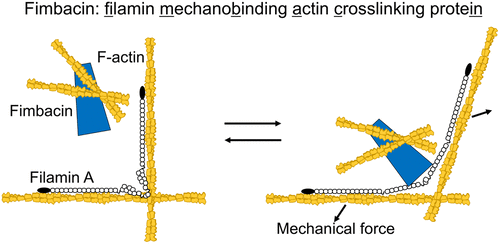
Filamin A (FLNA), an actin cross-linking protein, acts as a mechanosensor and mechanotransducer by exposing the cryptic binding site on repeat 21 (R21) to interact with integrin. Here, we investigated if any other biological molecule interacts with the cryptic binding site. Using proteomics and an in silico screening for a FLNA-binding motif, we identified and characterized a protein termed fimbacin (filamin mechanobinding actin cross-linking protein), encoded in the LUZP1 gene, as a novel FLNA-binding partner. Fimbacin does not interact with canonical full-length FLNA, but the exposure of a cryptic integrin-binding site of FLNA R21 enables fimbacin to interact. We have identified two FLNA binding sites on fimbacin and determined critical amino acid residues for the interaction. We also found that fimbacin itself is a new actin cross-linking protein and mapped the actin-binding site on amino acid residues 400–500. Fimbacin oligomerizes (estimated as an octamer on size exclusion chromatography) through the amino-terminal domain that is predicted to be a coiled-coil to cross-link actin filaments. When expressed, fimbacin localized to actin stress fibers in tissue culture cells. Although the interaction with FLNA is not necessary for fimbacin to colocalize with F-actin, fluorescent recovery after photobleaching (FRAP) revealed that their interaction stabilizes fimbacin on the actin cytoskeleton and that inhibition of Rho-kinase, an upstream activator of myosin II, also decreases the interaction presumably due to a loss of internal mechanical stress. Taken together, these data identify fimbacin as a new actin cross-linking protein that interacts with the FLNA mechanosensing domain R21.
中文翻译:

Filamin A机械结合伴侣II的鉴定:Fimbacin是一种新型肌动蛋白交联和Filamin A结合蛋白。
肌动蛋白交联蛋白Filamin A(FLNA)通过使重复序列21(R21)上的隐蔽结合位点与整联蛋白相互作用而充当机械传感器和机械转导子。在这里,我们研究了是否还有其他生物分子与隐蔽结合位点相互作用。使用蛋白质组学和计算机软件筛选FLNA结合基序,我们鉴定并鉴定了LUZP1基因中编码的称为fimbacin(纤维蛋白原机械结合肌动蛋白交联蛋白)的蛋白质,作为新型FLNA结合伴侣。Fimbacin不会与规范的全长FLNA相互作用,但是FLNA R21的隐伏整合素结合位点的暴露使Fmbacin能够相互作用。我们已经确定了fimbacin上的两个FLNA结合位点,并确定了相互作用的关键氨基酸残基。我们还发现,Fimbacin本身是一种新的肌动蛋白交联蛋白,并将肌动蛋白结合位点定位在400-500个氨基酸残基上。Fimbacin通过氨基末端结构域寡聚化(在尺寸排阻色谱法中估计为八聚体),该氨基末端结构域被认为是卷曲线圈与肌动蛋白丝交联。表达时 fimbacin定位于组织培养细胞中的肌动蛋白应激纤维。尽管与FLNA的相互作用对于纤维蛋白与F-肌动蛋白共定位不是必需的,但光漂白后的荧光恢复(FRAP)显示,它们的相互作用使纤维蛋白稳定在肌动蛋白细胞骨架上,并且抑制了肌球蛋白II的上游激活剂Rho激酶。可能是由于内部机械应力的损失而降低了相互作用。综上所述,这些数据将fimbacin鉴定为一种新的肌动蛋白交联蛋白,可与FLNA机械传感结构域R21相互作用。光漂白后的荧光恢复(FRAP)表明,它们的相互作用使纤维蛋白稳定在肌动蛋白的细胞骨架上,抑制Rho激酶(肌球蛋白II的上游激活剂)也降低了相互作用,可能是由于内部机械应力的损失。综上所述,这些数据将fimbacin鉴定为一种新的肌动蛋白交联蛋白,可与FLNA机械传感域R21相互作用。光漂白后的荧光恢复(FRAP)表明,它们的相互作用使纤维蛋白稳定在肌动蛋白的细胞骨架上,抑制Rho激酶(肌球蛋白II的上游激活剂)也降低了相互作用,可能是由于内部机械应力的损失。综上所述,这些数据将fimbacin鉴定为一种新的肌动蛋白交联蛋白,可与FLNA机械传感域R21相互作用。
更新日期:2019-11-18
中文翻译:

Filamin A机械结合伴侣II的鉴定:Fimbacin是一种新型肌动蛋白交联和Filamin A结合蛋白。
肌动蛋白交联蛋白Filamin A(FLNA)通过使重复序列21(R21)上的隐蔽结合位点与整联蛋白相互作用而充当机械传感器和机械转导子。在这里,我们研究了是否还有其他生物分子与隐蔽结合位点相互作用。使用蛋白质组学和计算机软件筛选FLNA结合基序,我们鉴定并鉴定了LUZP1基因中编码的称为fimbacin(纤维蛋白原机械结合肌动蛋白交联蛋白)的蛋白质,作为新型FLNA结合伴侣。Fimbacin不会与规范的全长FLNA相互作用,但是FLNA R21的隐伏整合素结合位点的暴露使Fmbacin能够相互作用。我们已经确定了fimbacin上的两个FLNA结合位点,并确定了相互作用的关键氨基酸残基。我们还发现,Fimbacin本身是一种新的肌动蛋白交联蛋白,并将肌动蛋白结合位点定位在400-500个氨基酸残基上。Fimbacin通过氨基末端结构域寡聚化(在尺寸排阻色谱法中估计为八聚体),该氨基末端结构域被认为是卷曲线圈与肌动蛋白丝交联。表达时 fimbacin定位于组织培养细胞中的肌动蛋白应激纤维。尽管与FLNA的相互作用对于纤维蛋白与F-肌动蛋白共定位不是必需的,但光漂白后的荧光恢复(FRAP)显示,它们的相互作用使纤维蛋白稳定在肌动蛋白细胞骨架上,并且抑制了肌球蛋白II的上游激活剂Rho激酶。可能是由于内部机械应力的损失而降低了相互作用。综上所述,这些数据将fimbacin鉴定为一种新的肌动蛋白交联蛋白,可与FLNA机械传感结构域R21相互作用。光漂白后的荧光恢复(FRAP)表明,它们的相互作用使纤维蛋白稳定在肌动蛋白的细胞骨架上,抑制Rho激酶(肌球蛋白II的上游激活剂)也降低了相互作用,可能是由于内部机械应力的损失。综上所述,这些数据将fimbacin鉴定为一种新的肌动蛋白交联蛋白,可与FLNA机械传感域R21相互作用。光漂白后的荧光恢复(FRAP)表明,它们的相互作用使纤维蛋白稳定在肌动蛋白的细胞骨架上,抑制Rho激酶(肌球蛋白II的上游激活剂)也降低了相互作用,可能是由于内部机械应力的损失。综上所述,这些数据将fimbacin鉴定为一种新的肌动蛋白交联蛋白,可与FLNA机械传感域R21相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号