Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enabling cell recovery from 3D cell culture microfluidic devices for tumour microenvironment biomarker profiling.
Scientific Reports ( IF 3.8 ) Pub Date : 2019-04-17 , DOI: 10.1038/s41598-019-42529-8 María Virumbrales-Muñoz 1 , Jose M Ayuso 1, 2 , Alodia Lacueva 3, 4, 5 , Teodora Randelovic 3, 4, 5 , Megan K Livingston 1, 6 , David J Beebe 1, 7 , Sara Oliván 3, 4, 5 , Desirée Pereboom 8 , Manuel Doblare 3, 4, 5 , Luis Fernández 3, 4, 5 , Ignacio Ochoa 3, 4, 5
Scientific Reports ( IF 3.8 ) Pub Date : 2019-04-17 , DOI: 10.1038/s41598-019-42529-8 María Virumbrales-Muñoz 1 , Jose M Ayuso 1, 2 , Alodia Lacueva 3, 4, 5 , Teodora Randelovic 3, 4, 5 , Megan K Livingston 1, 6 , David J Beebe 1, 7 , Sara Oliván 3, 4, 5 , Desirée Pereboom 8 , Manuel Doblare 3, 4, 5 , Luis Fernández 3, 4, 5 , Ignacio Ochoa 3, 4, 5
Affiliation
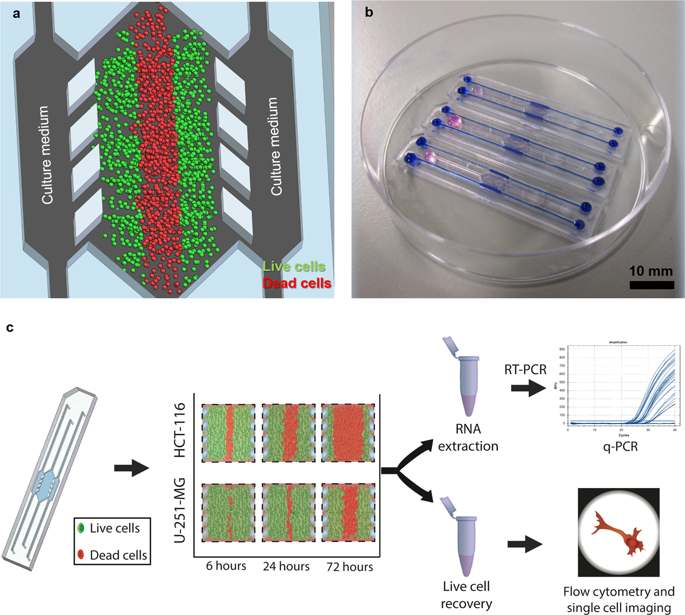
|
The tumour microenvironment (TME) has recently drawn much attention due to its profound impact on tumour development, drug resistance and patient outcome. There is an increasing interest in new therapies that target the TME. Nonetheless, most established in vitro models fail to include essential cues of the TME. Microfluidics can be used to reproduce the TME in vitro and hence provide valuable insight on tumour evolution and drug sensitivity. However, microfluidics remains far from well-established mainstream molecular and cell biology methods. Therefore, we have developed a quick and straightforward collagenase-based enzymatic method to recover cells embedded in a 3D hydrogel in a microfluidic device with no impact on cell viability. We demonstrate the validity of this method on two different cell lines in a TME microfluidic model. Cells were successfully retrieved with high viability, and we characterised the different cell death mechanisms via AMNIS image cytometry in our model.
中文翻译:

支持从3D细胞培养微流控设备恢复细胞以进行肿瘤微环境生物标志物分析。
肿瘤微环境(TME)由于其对肿瘤发展,耐药性和患者预后的深远影响而受到了广泛关注。人们越来越关注针对TME的新疗法。但是,大多数已建立的体外模型均未包含TME的基本提示。微流控技术可用于体外复制TME,因此可提供有关肿瘤演变和药物敏感性的宝贵见解。然而,微流控技术还远未建立成熟的主流分子和细胞生物学方法。因此,我们开发了一种快速,直接的基于胶原酶的酶法,可回收嵌入微流体设备中3D水凝胶中的细胞,而对细胞生存力没有影响。我们在TME微流体模型中的两个不同的细胞系上证明了此方法的有效性。
更新日期:2019-04-17
中文翻译:

支持从3D细胞培养微流控设备恢复细胞以进行肿瘤微环境生物标志物分析。
肿瘤微环境(TME)由于其对肿瘤发展,耐药性和患者预后的深远影响而受到了广泛关注。人们越来越关注针对TME的新疗法。但是,大多数已建立的体外模型均未包含TME的基本提示。微流控技术可用于体外复制TME,因此可提供有关肿瘤演变和药物敏感性的宝贵见解。然而,微流控技术还远未建立成熟的主流分子和细胞生物学方法。因此,我们开发了一种快速,直接的基于胶原酶的酶法,可回收嵌入微流体设备中3D水凝胶中的细胞,而对细胞生存力没有影响。我们在TME微流体模型中的两个不同的细胞系上证明了此方法的有效性。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号