当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile Preparation of Spirolactones by an Alkoxycarbonyl Radical Cyclization-Cross-Coupling Cascade.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-05-13 , DOI: 10.1002/anie.201903353 Nicholas A Weires 1 , Yuriy Slutskyy 1, 2 , Larry E Overman 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-05-13 , DOI: 10.1002/anie.201903353 Nicholas A Weires 1 , Yuriy Slutskyy 1, 2 , Larry E Overman 1
Affiliation
|
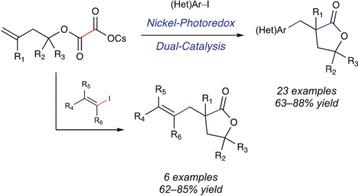
An alkoxycarbonyl radical cyclization–cross‐coupling cascade has been developed that allows functionalized γ‐butyrolactones to be prepared in one step from simple tertiary alcohol‐derived homoallylic oxalate precursors. The reaction succeeds with aryl and vinyl electrophiles and is compatible with heterocyclic fragments in both coupling partners. This chemistry allows for the rapid construction of spirolactones, which are of interest in drug discovery endeavors.
中文翻译:

通过烷氧羰基自由基环化-交叉偶联级联轻松制备螺内酯。
已开发出烷氧基羰基自由基环化-交叉偶联级联反应,可从简单的叔醇衍生的均芳草酸均聚物前体一步制备官能化的γ-丁内酯。该反应在芳基和乙烯基亲电子试剂上成功完成,并且与两个偶联配偶体中的杂环片段均相容。这种化学性质使得螺内酯的快速构建成为可能,这在药物开发中很有意义。
更新日期:2019-05-13
中文翻译:

通过烷氧羰基自由基环化-交叉偶联级联轻松制备螺内酯。
已开发出烷氧基羰基自由基环化-交叉偶联级联反应,可从简单的叔醇衍生的均芳草酸均聚物前体一步制备官能化的γ-丁内酯。该反应在芳基和乙烯基亲电子试剂上成功完成,并且与两个偶联配偶体中的杂环片段均相容。这种化学性质使得螺内酯的快速构建成为可能,这在药物开发中很有意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号