Scientific Reports ( IF 3.8 ) Pub Date : 2019-04-16 , DOI: 10.1038/s41598-019-42524-z Laura Acquasaliente , Leslie A. Pelc , Enrico Di Cera
|
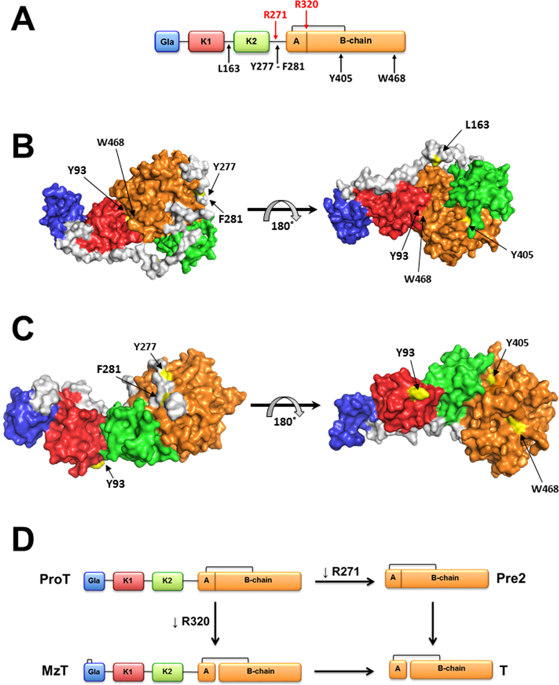
Prothrombin, or coagulation factor II, is a multidomain zymogen precursor of thrombin that undergoes an allosteric equilibrium between two alternative conformations, open and closed, that react differently with the physiological activator prothrombinase. Specifically, the dominant closed form promotes cleavage at R320 and initiates activation along the meizothrombin pathway, whilst the open form promotes cleavage at R271 and initiates activation along the alternative prethrombin-2 pathway. Here we report how key structural features of prothrombin can be monitored by limited proteolysis with chymotrypsin that attacks W468 in the flexible autolysis loop of the protease domain in the open but not the closed form. Perturbation of prothrombin by selective removal of its constituent Gla domain, kringles and linkers reveals their long-range communication and supports a scenario where stabilization of the open form switches the pathway of activation from meizothrombin to prethrombin-2. We also identify R296 in the A chain of the protease domain as a critical link between the allosteric open-closed equilibrium and exposure of the sites of cleavage at R271 and R320. These findings reveal important new details on the molecular basis of prothrombin function.
中文翻译:

通过有限的蛋白水解探测凝血酶原结构
凝血酶原或凝血因子II是凝血酶的多域酶原前体,它在两个替代构象(开放和闭合构象)之间发生变构平衡,并与生理活化剂凝血酶原酶发生不同反应。具体而言,显性闭合形式促进在R320处的裂解并沿中纤溶酶途径启动激活,而开放形式促进在R271处的裂解并沿替代性凝血酶原2途径启动激活。在这里我们报告凝血酶原的关键结构特征如何通过胰凝乳蛋白酶的有限蛋白水解来监测,所述胰凝乳蛋白酶以开放而不是封闭的形式攻击蛋白酶域的柔性自溶环中的W468。通过选择性去除其组成部分的Gla结构域来干扰凝血酶原,kringles和接头显示了它们的远程通讯,并支持以下情形:开放形式的稳定将活化途径从meothothrombin转换为prethrombin-2。我们还将蛋白酶结构域A链中的R296识别为变构开闭平衡与R271和R320切割位点的暴露之间的关键链接。这些发现揭示了凝血酶原功能的分子基础上的重要新细节。






























 京公网安备 11010802027423号
京公网安备 11010802027423号