当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Extraction of Lithium Ion from Aqueous Solution with Sodium Phosphomolybdate As a Coextraction Agent
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-04-15 00:00:00 , DOI: 10.1021/acssuschemeng.9b00898 Zhiyong Zhou 1 , Haotian Liu 1 , Jiahui Fan 1 , Xueting Liu 1 , Yafei Hu 1 , Yulei Hu 1 , Yong Wang 1 , Zhongqi Ren 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-04-15 00:00:00 , DOI: 10.1021/acssuschemeng.9b00898 Zhiyong Zhou 1 , Haotian Liu 1 , Jiahui Fan 1 , Xueting Liu 1 , Yafei Hu 1 , Yulei Hu 1 , Yong Wang 1 , Zhongqi Ren 1
Affiliation
|
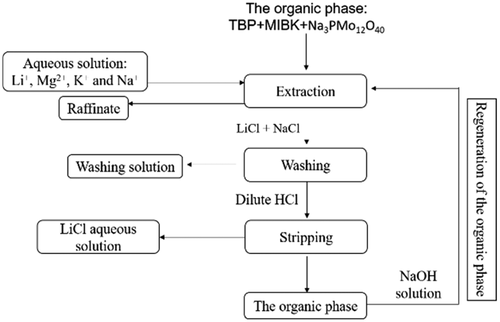
Sodium phosphomolybdate with a spatial stereo symmetry structure and stability in acid and alkali was used as a new coextraction agent for separating Li+. In addition, methyl isobutyl ketone and tributyl phosphate were used as diluent and extractant, respectively. The influence of operation conditions on separation performance of lithium ion was investigated. The single stage extraction efficiency of Li+ was 58.74% with the volume fraction of tributyl phosphate, O/A phase ratio, and molar ratio of sodium phosphomolybdate to lithium as 40%, 3.6, and 1, respectively. Then the cross-flow and saturated extraction experiments were conducted. When 0.2 mol/L LiCl + 1.8 mol/L NaCl solution was used as washing agent and O/A phase ratio was 3, the washing efficiency of Mg2+ reached up to 99.59%. When 0.26 mol/L HCl solution was used as stripping agent and O/A phase ratio was 3, the stripping efficiency of Li+ reached 98.3%. The difference in extraction efficiency was within 6.5% through 10 recyclings of the organic phase, indicating that the coextraction agent was very stable. The extraction mechanism was investigated and the binding ability of P═O in tributyl phosphate molecule followed the sequence Li+ ≫ Na+ > K+ > Mg2+.
中文翻译:

磷钼酸钠作为共萃取剂从水溶液中选择性萃取锂离子
具有空间立体对称性和在酸碱中稳定的磷钼酸钠被用作分离Li +的新的共萃取剂。另外,甲基异丁基酮和磷酸三丁酯分别用作稀释剂和萃取剂。研究了操作条件对锂离子分离性能的影响。Li +的单级萃取效率为58.74%,磷酸三丁酯的体积分数,O / A相比和磷钼酸钠与锂的摩尔比分别为40%,3.6和1。然后进行错流和饱和萃取实验。当使用0.2 mol / L LiCl + 1.8 mol / L NaCl溶液作为清洗剂且O / A相比为3时,Mg 2+的清洗效率达到了99.59%。当使用0.26 mol / L HCl溶液作为汽提剂,O / A相比为3时,Li +的汽提效率达到98.3%。通过有机相的10次循环,萃取效率的差异在6.5%以内,表明共萃取剂非常稳定。研究了其提取机理,磷酸三丁酯分子中P═O的结合能力遵循Li + + Na + > K + > Mg 2+的顺序。
更新日期:2019-04-15
中文翻译:

磷钼酸钠作为共萃取剂从水溶液中选择性萃取锂离子
具有空间立体对称性和在酸碱中稳定的磷钼酸钠被用作分离Li +的新的共萃取剂。另外,甲基异丁基酮和磷酸三丁酯分别用作稀释剂和萃取剂。研究了操作条件对锂离子分离性能的影响。Li +的单级萃取效率为58.74%,磷酸三丁酯的体积分数,O / A相比和磷钼酸钠与锂的摩尔比分别为40%,3.6和1。然后进行错流和饱和萃取实验。当使用0.2 mol / L LiCl + 1.8 mol / L NaCl溶液作为清洗剂且O / A相比为3时,Mg 2+的清洗效率达到了99.59%。当使用0.26 mol / L HCl溶液作为汽提剂,O / A相比为3时,Li +的汽提效率达到98.3%。通过有机相的10次循环,萃取效率的差异在6.5%以内,表明共萃取剂非常稳定。研究了其提取机理,磷酸三丁酯分子中P═O的结合能力遵循Li + + Na + > K + > Mg 2+的顺序。

































 京公网安备 11010802027423号
京公网安备 11010802027423号