当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-Mediated Dehydrogenative Borylation–Hydroborylation of Bis(alkyl)alkynes: Intermediates and Mechanism
Organometallics ( IF 2.5 ) Pub Date : 2019-04-12 , DOI: 10.1021/acs.organomet.9b00104 Sheila G. Curto 1 , Miguel A. Esteruelas 1 , Montserrat Oliván 1 , Enrique Oñate 1
Organometallics ( IF 2.5 ) Pub Date : 2019-04-12 , DOI: 10.1021/acs.organomet.9b00104 Sheila G. Curto 1 , Miguel A. Esteruelas 1 , Montserrat Oliván 1 , Enrique Oñate 1
Affiliation
|
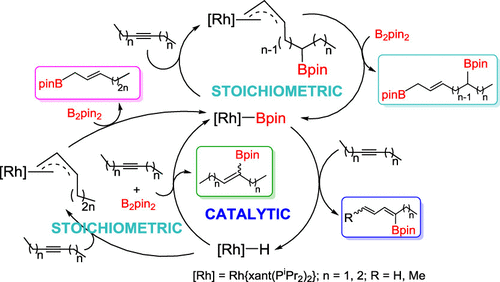
Complex Rh(Bpin){κ3-P,O,P-[xant(PiPr2)2]} (Bpin = pinacolboryl; xant(PiPr2)2 = 9,9-dimethyl-4,5-bis(diisopropylphosphino)xanthene) catalyzes the addition of B2pin2 to 3-hexyne and 4-octyne to give equimolecular mixtures of conjugated boryldienes and borylolefins, as a result of the addition of the B–B bond of the diborane to different molecules of alkynes and hydride transfer from one to the other. Both the dehydrogenative borylation and hydroborylation reactions form a catalytic cycle that has been deduced on the basis of stoichiometric studies. Complex Rh(Bpin){κ3-P,O,P-[xant(PiPr2)2]} promotes the dehydrogenative borylation of alkynes by means of reactions of insertion of the alkyne into the Rh–B bond, Z–E isomerization of the β-borylalkenyl ligand of the resulting Rh–alkenyl species, and Cγ–H bond activation of the alkyl substituent attached to the alkenyl Cα atom. As a consequence of the formation of boryldienes, the monohydride RhH{κ3-P,O,P-[xant(PiPr2)2]} is generated. The latter in a sequential manner reacts with the alkynes and the diborane to give the borylolefin hydroborylation products, via Rh–alkenyl intermediates, and regenerates the initial Rh–boryl compound. The latter also promotes stoichiometric cycles to prepare diboryl-2-olefins via allyl intermediates. In addition, the stoichiometric rhodium-mediated formation of 1-boryl-2-olefins is shown.
中文翻译:

铑介导的双(烷基)炔烃的脱氢硼氢化-加氢硼化:中间体和机理
复杂的Rh(BPIN){κ 3 -P,O,P- [xant(P我镨2)2 ]}(BPIN = pinacolboryl; xant(P我镨2)2 = 9,9-二甲基-4,5-双(二异丙基膦基)x吨)催化将B 2销2加到3-己炔和4-辛炔中,得到共轭硼烷基二烯和硼烷基烯烃的等分子混合物,这是由于乙硼烷的B–B键添加到不同分子上的结果炔烃和氢化物从一个转移到另一个。脱氢硼酸酯化反应和氢硼酸化反应均形成催化循环,该催化循环是根据化学计量研究推导的。复杂的Rh(BPIN){κ 3 -P,O,P- [xant(P我镨2) 2 ]}由炔入的Rh-B键的插入反应的手段促进炔烃的脱氢硼基化, ž - ë所得的Rh-烯基物质的β-borylalkenyl配体的异构化,和C γ连接至烯基Cα原子的烷基取代基的-H键活化。作为boryldienes的形成的结果,一氢化RhH的{κ 3 -P,O,P- [xant(P我镨2) 2]}生成。后者以顺序的方式与炔烃和乙硼烷反应,通过Rh-烯基中间体,得到硼烷基烯烃加氢硼化产物,并再生了最初的Rh-硼基化合物。后者还促进化学计量循环,以通过烯丙基中间体制备二硼烷基-2-烯烃。另外,显示了化学计量的铑介导的1-硼烷基-2-烯烃的形成。
更新日期:2019-04-15
中文翻译:

铑介导的双(烷基)炔烃的脱氢硼氢化-加氢硼化:中间体和机理
复杂的Rh(BPIN){κ 3 -P,O,P- [xant(P我镨2)2 ]}(BPIN = pinacolboryl; xant(P我镨2)2 = 9,9-二甲基-4,5-双(二异丙基膦基)x吨)催化将B 2销2加到3-己炔和4-辛炔中,得到共轭硼烷基二烯和硼烷基烯烃的等分子混合物,这是由于乙硼烷的B–B键添加到不同分子上的结果炔烃和氢化物从一个转移到另一个。脱氢硼酸酯化反应和氢硼酸化反应均形成催化循环,该催化循环是根据化学计量研究推导的。复杂的Rh(BPIN){κ 3 -P,O,P- [xant(P我镨2) 2 ]}由炔入的Rh-B键的插入反应的手段促进炔烃的脱氢硼基化, ž - ë所得的Rh-烯基物质的β-borylalkenyl配体的异构化,和C γ连接至烯基Cα原子的烷基取代基的-H键活化。作为boryldienes的形成的结果,一氢化RhH的{κ 3 -P,O,P- [xant(P我镨2) 2]}生成。后者以顺序的方式与炔烃和乙硼烷反应,通过Rh-烯基中间体,得到硼烷基烯烃加氢硼化产物,并再生了最初的Rh-硼基化合物。后者还促进化学计量循环,以通过烯丙基中间体制备二硼烷基-2-烯烃。另外,显示了化学计量的铑介导的1-硼烷基-2-烯烃的形成。















































 京公网安备 11010802027423号
京公网安备 11010802027423号