当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Viedma Ripening of Chiral Coordination Polymers Based on Achiral Molecules
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2019-04-15 00:00:00 , DOI: 10.1021/acs.cgd.9b00235 Shu-Ting Wu 1, 2 , Yu-Sheng Zhang 1 , Bin Zhang 1 , Xiao-Lin Hu 1 , Xi-He Huang 1 , Chang-Cang Huang 1 , Nai-Feng Zhuang 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2019-04-15 00:00:00 , DOI: 10.1021/acs.cgd.9b00235 Shu-Ting Wu 1, 2 , Yu-Sheng Zhang 1 , Bin Zhang 1 , Xiao-Lin Hu 1 , Xi-He Huang 1 , Chang-Cang Huang 1 , Nai-Feng Zhuang 1
Affiliation
|
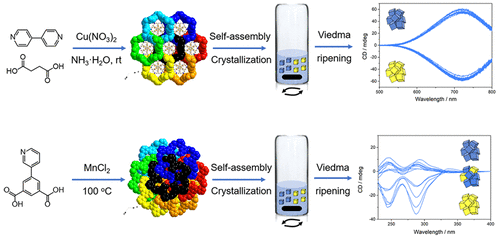
When the chiral coordination polymers were composed of achiral molecules, the deracemization of the bulk product remains a great challenge if without any chiral induction. The Viedma ripening theory was adopted herein to help the achievement of mirror symmetry breaking for the bulk chirality of two chiral coordination polymers. The enantiomeric excess of the bulk chirality for the first compound is about 100%, which means absolute asymmetric synthesis. The deracemization degree of the bulk chirality for the second compound fluctuates occasionally. Further study has discussed the different deracemization degree of the bulk chirality for two compounds with the consideration of enantioselective incorporation in crystal cluster.
中文翻译:

基于手性分子的手性配位聚合物的前驱成熟
当手性配位聚合物由非手性分子组成时,如果没有任何手性诱导,则本体产物的脱硫仍然是巨大的挑战。本文采用Viedma成熟理论来帮助实现两种手性配位聚合物的整体手性的镜像对称性破坏。第一种化合物的手性的对映异构体过量约为100%,这意味着绝对不对称合成。第二化合物的本体手性的脱消旋度偶尔波动。进一步的研究讨论了考虑到对映体选择性结合到晶体团簇中两种化合物的不同手性的脱消臭度。
更新日期:2019-04-15
中文翻译:

基于手性分子的手性配位聚合物的前驱成熟
当手性配位聚合物由非手性分子组成时,如果没有任何手性诱导,则本体产物的脱硫仍然是巨大的挑战。本文采用Viedma成熟理论来帮助实现两种手性配位聚合物的整体手性的镜像对称性破坏。第一种化合物的手性的对映异构体过量约为100%,这意味着绝对不对称合成。第二化合物的本体手性的脱消旋度偶尔波动。进一步的研究讨论了考虑到对映体选择性结合到晶体团簇中两种化合物的不同手性的脱消臭度。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号