当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Xylo-C-nucleosides with a pyrrolo[2,1-f][1,2,4]triazin-4-amine heterocyclic base: Synthesis and antiproliferative properties.
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-04-15 , DOI: 10.1016/j.bmcl.2019.04.023 Peng Nie 1 , Elisabetta Groaz 1 , Dirk Daelemans 2 , Piet Herdewijn 1
Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-04-15 , DOI: 10.1016/j.bmcl.2019.04.023 Peng Nie 1 , Elisabetta Groaz 1 , Dirk Daelemans 2 , Piet Herdewijn 1
Affiliation
|
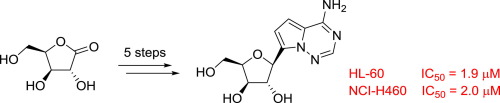
The synthesis of a xylo-C-nucleoside containing pyrrolo[2,1-f][1,2,4]triazin-4-amine as nucleobase along with that of its 1'-cyano analogue is described. Among different experimental conditions explored in order to optimize a key debenzylation step in the presented synthetic route, it was found that palladium catalyzed hydrogen transfer allowed for obtaining the target compounds in good yields. The resulting mixture of epimers was separated and each was characterized by NOESY NMR experiments. In vitro antiproliferative assays showed that the 1'-unsubstituted analogue was active against a panel of tumor cell lines such as the human leukemia HL-60 (IC50 = 1.9 µM) and lung cancer NCI-H460 (IC50 = 2.0 µM) cells.
中文翻译:

具有吡咯并[2,1-f] [1,2,4]三嗪-4胺杂环碱基的木糖-C-核苷:合成和抗增殖特性。
描述了含有吡咯并[2,1-f] [1,2,4]三嗪-4-胺作为核碱基的xylo-C-核苷及其1'-氰基类似物的合成。在为优化所提出的合成路线中的关键脱苄基步骤而探索的不同实验条件中,发现钯催化的氢转移可以以高收率获得目标化合物。分离得到的差向异构体混合物,并通过NOESY NMR实验对其进行表征。体外抗增殖试验表明1'-未取代的类似物对一组肿瘤细胞系具有活性,例如人类白血病HL-60(IC50 = 1.9 µM)和肺癌NCI-H460(IC50 = 2.0 µM)细胞。
更新日期:2019-04-15
中文翻译:

具有吡咯并[2,1-f] [1,2,4]三嗪-4胺杂环碱基的木糖-C-核苷:合成和抗增殖特性。
描述了含有吡咯并[2,1-f] [1,2,4]三嗪-4-胺作为核碱基的xylo-C-核苷及其1'-氰基类似物的合成。在为优化所提出的合成路线中的关键脱苄基步骤而探索的不同实验条件中,发现钯催化的氢转移可以以高收率获得目标化合物。分离得到的差向异构体混合物,并通过NOESY NMR实验对其进行表征。体外抗增殖试验表明1'-未取代的类似物对一组肿瘤细胞系具有活性,例如人类白血病HL-60(IC50 = 1.9 µM)和肺癌NCI-H460(IC50 = 2.0 µM)细胞。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号