Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Adversarial DNA N6-Methyladenine-Sensor Network Preserves Polycomb Silencing.
Molecular Cell ( IF 14.5 ) Pub Date : 2019-04-11 , DOI: 10.1016/j.molcel.2019.03.018 Soo-Mi Kweon 1 , Yibu Chen 2 , Eugene Moon 1 , Kotryna Kvederaviciutė 3 , Saulius Klimasauskas 3 , Douglas E Feldman 1
Molecular Cell ( IF 14.5 ) Pub Date : 2019-04-11 , DOI: 10.1016/j.molcel.2019.03.018 Soo-Mi Kweon 1 , Yibu Chen 2 , Eugene Moon 1 , Kotryna Kvederaviciutė 3 , Saulius Klimasauskas 3 , Douglas E Feldman 1
Affiliation
|
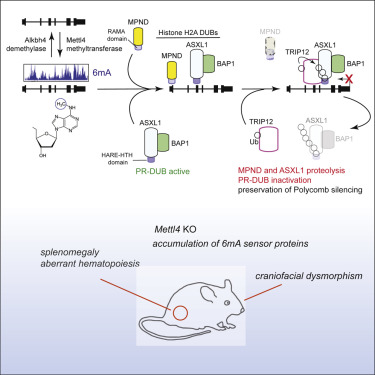
Adenine N6 methylation in DNA (6mA) is widespread among bacteria and phage and is detected in mammalian genomes, where its function is largely unexplored. Here we show that 6mA deposition and removal are catalyzed by the Mettl4 methyltransferase and Alkbh4 dioxygenase, respectively, and that 6mA accumulation in genic elements corresponds with transcriptional silencing. Inactivation of murine Mettl4 depletes 6mA and causes sublethality and craniofacial dysmorphism in incross progeny. We identify distinct 6mA sensor domains of prokaryotic origin within the MPND deubiquitinase and ASXL1, a component of the Polycomb repressive deubiquitinase (PR-DUB) complex, both of which act to remove monoubiquitin from histone H2A (H2A-K119Ub), a repressive mark. Deposition of 6mA by Mettl4 triggers the proteolytic destruction of both sensor proteins, preserving genome-wide H2A-K119Ub levels. Expression of the bacterial 6mA methyltransferase Dam, in contrast, fails to destroy either sensor. These findings uncover a native, adversarial 6mA network architecture that preserves Polycomb silencing.
中文翻译:

对抗性DNA N6-甲基腺嘌呤传感器网络保留了多梳沉默。
DNA(6mA)中的腺嘌呤N6甲基化在细菌和噬菌体中广泛存在,并在哺乳动物基因组中被检测到,在该基因组中其功能尚未得到充分开发。在这里,我们显示6mA沉积和去除分别由Mettl4甲基转移酶和Alkbh4双加氧酶催化,并且6mA在基因元件中的积累与转录沉默相对应。小鼠Mettl4的失活消耗了6mA电流,并在异代子代中引起亚致死性和颅面畸形。我们在MPND去泛素酶和ASXL1(多梳抑制性泛素化酶(PR-DUB)复合物的组成部分)中鉴定了原核起源的不同6mA传感器域,两者均起着从组蛋白H2A(H2A-K119Ub)(一种抑制性标记)中去除单泛素的作用。Mettl4沉积6mA会触发两种传感器蛋白的蛋白水解破坏,保持全基因组H2A-K119Ub的水平。相反,细菌6mA甲基转移酶Dam的表达不能破坏任何一个传感器。这些发现揭示了一种本机的,对抗性的6mA网络架构,该架构保留了Polycomb沉默。
更新日期:2019-05-16
中文翻译:

对抗性DNA N6-甲基腺嘌呤传感器网络保留了多梳沉默。
DNA(6mA)中的腺嘌呤N6甲基化在细菌和噬菌体中广泛存在,并在哺乳动物基因组中被检测到,在该基因组中其功能尚未得到充分开发。在这里,我们显示6mA沉积和去除分别由Mettl4甲基转移酶和Alkbh4双加氧酶催化,并且6mA在基因元件中的积累与转录沉默相对应。小鼠Mettl4的失活消耗了6mA电流,并在异代子代中引起亚致死性和颅面畸形。我们在MPND去泛素酶和ASXL1(多梳抑制性泛素化酶(PR-DUB)复合物的组成部分)中鉴定了原核起源的不同6mA传感器域,两者均起着从组蛋白H2A(H2A-K119Ub)(一种抑制性标记)中去除单泛素的作用。Mettl4沉积6mA会触发两种传感器蛋白的蛋白水解破坏,保持全基因组H2A-K119Ub的水平。相反,细菌6mA甲基转移酶Dam的表达不能破坏任何一个传感器。这些发现揭示了一种本机的,对抗性的6mA网络架构,该架构保留了Polycomb沉默。































 京公网安备 11010802027423号
京公网安备 11010802027423号