Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-04-11 , DOI: 10.1016/j.tetlet.2019.04.005 Hongru Dong , Yuming Zhao
|
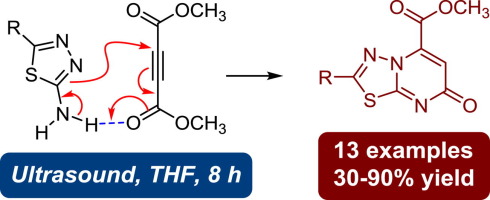
7-Oxo-7H-[1,3,4]thiadiazolo[3,2-a]pyrimidine-5-carboxylate derivatives are biologically and pharmacologically useful heterocycles. An efficient synthetic methodology for this class of compounds was developed through catalyst-free, one-pot reactions between 2-aminothiadiazoles and dimethyl acetylenedicarboxylate (DMAD) in THF with the aid of ultrasound irradiation. The reactions show applicability to a wide range of substrates and high regioselectivity for the “7-one” products over their “5-one” isomers. Detailed reaction mechanisms were mapped out by theoretical modeling analysis based on density functional theory (DFT) calculations. Mechanistic studies indicate that the favored reaction pathway involves a sequence of hydrogen-bond directed Michael addition, synergistic proton transfer/five-membered ring opening, and intramolecular cyano hetero-Diels-Alder reactions.
中文翻译:

在温和条件下高度区域选择性合成7-oxo-7 H- [1,3,4]噻二唑[3,2 - a ]嘧啶-5-羧酸酯衍生物
7-Oxo-7 H- [1,3,4]噻二唑[3,2- a]嘧啶-5-羧酸酯衍生物是生物学上和药理上有用的杂环。通过在超声波辐射的帮助下,2-氨基噻二唑与乙炔二羧酸二甲酯(DMAD)在THF中进行无催化剂的一锅反应,开发出了用于此类化合物的有效合成方法。该反应显示出适用于多种底物,并且相对于其“ 5-one”异构体,“ 7-one”产物具有较高的区域选择性。通过基于密度泛函理论(DFT)计算的理论模型分析,绘制出详细的反应机理。机理研究表明,有利的反应途径包括一系列氢键引导的迈克尔加成,协同质子转移/五元开环和分子内氰基杂-Diels-Alder反应。






























 京公网安备 11010802027423号
京公网安备 11010802027423号