当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potassium‐Persulfate‐Promoted Regioselective Azidation/Cyclization of 1,6‐Enynes
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-04-12 , DOI: 10.1002/ajoc.201900210 Yi‐Ning Wang 1 , Qiang Li 2 , Yi‐Lin Fang 3 , Le‐Han Gao 1 , Si‐Zhe Song 1 , Youren Dong 1 , Wen‐Ting Wei 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-04-12 , DOI: 10.1002/ajoc.201900210 Yi‐Ning Wang 1 , Qiang Li 2 , Yi‐Lin Fang 3 , Le‐Han Gao 1 , Si‐Zhe Song 1 , Youren Dong 1 , Wen‐Ting Wei 1
Affiliation
|
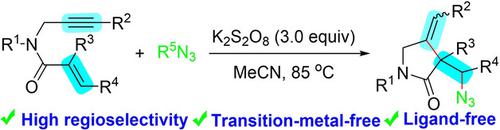
An efficient, environmentally benign and sustainable method for the synthesis of 2‐pyrrolidinones from 1,6‐enynes via an unusual regioselective azidation/cyclization is described. The products have been obtained in good yields without the assistance of any transition‐metal catalysts or ligands. To demonstrate the utility of this protocol, the azidation products are converted to a synthetically valuable amine or triazole. This azidation cyclization involves a radical process, allows a regioselective way toward the formation of one C−N bond and one C−C bond.
中文翻译:

过硫酸钾促进的1,6-烯炔的区域选择性叠氮化/环化
描述了一种通过不寻常的区域选择性叠氮化/环化反应从1,6-烯炔合成2-吡咯烷酮的高效,环境友好和可持续的方法。无需任何过渡金属催化剂或配体的辅助,即可获得高收率的产品。为了证明该协议的实用性,将叠氮化产物转化为合成上有价值的胺或三唑。该叠氮化环化涉及一个自由基过程,允许区域选择性地形成一个C-N键和一个C-C键。
更新日期:2019-04-12
中文翻译:

过硫酸钾促进的1,6-烯炔的区域选择性叠氮化/环化
描述了一种通过不寻常的区域选择性叠氮化/环化反应从1,6-烯炔合成2-吡咯烷酮的高效,环境友好和可持续的方法。无需任何过渡金属催化剂或配体的辅助,即可获得高收率的产品。为了证明该协议的实用性,将叠氮化产物转化为合成上有价值的胺或三唑。该叠氮化环化涉及一个自由基过程,允许区域选择性地形成一个C-N键和一个C-C键。































 京公网安备 11010802027423号
京公网安备 11010802027423号