当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How Do Size and Aggregation of Ice-binding Proteins Control their Ice Nucleation Efficiency
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-12 , DOI: 10.1021/jacs.9b01854 Yuqing Qiu 1 , Arpa Hudait 1 , Valeria Molinero 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-12 , DOI: 10.1021/jacs.9b01854 Yuqing Qiu 1 , Arpa Hudait 1 , Valeria Molinero 1
Affiliation
|
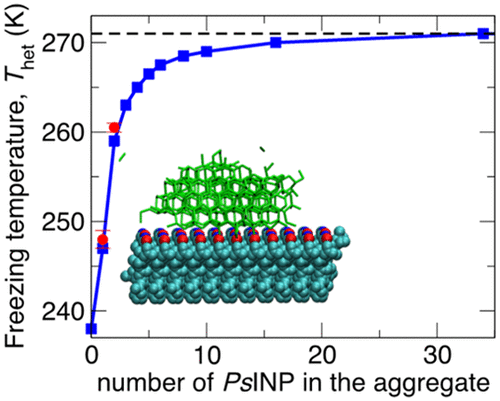
Organisms that thrive at cold temperatures produce ice-binding proteins to manage the nucleation and growth of ice. Bacterial ice-nucleating proteins (INP) are typically large and form aggregates in the cell membrane, while insect hyperactive antifreeze proteins (AFP) are soluble and generally small. Experiments indicate that larger ice-binding proteins and their aggregates nucleate ice at warmer temperatures. Nevertheless, a quantitative understanding of how size and aggregation of ice-binding proteins determine the temperature Thet at which proteins nucleate ice is still lacking. Here, we address this question using molecular simulations and nucleation theory. The simulations indicate that the 2.5 nm long antifreeze protein TmAFP nucleates ice at 2 ± 1 °C above the homogeneous nucleation temperature, in good agreement with recent experiments. We predict that the addition of ice-binding loops to TmAFP increases Thet, but not enough to compete in efficiency with the bacterial INP. We implement an accurate procedure to determine Thet of surfaces of finite size using classical nucleation theory, and, after validating the theory against Thet of the proteins in molecular simulations, we use it to predict Thet of the INP of Ps. syringae as a function of the length and number of proteins in the aggregates. We conclude that assemblies with at most 34 INP already reach the Thet = -2 °C characteristic of this bacterium. Interestingly, we find that Thet is a strongly varying nonmonotonic function of the distance between proteins in the aggregates. This indicates that, to achieve maximum freezing efficiency, bacteria must exert exquisite, subangstrom control of the distance between INP in their membrane.
中文翻译:

冰结合蛋白的大小和聚集如何控制它们的冰成核效率
在低温下茁壮成长的生物会产生冰结合蛋白来控制冰的成核和生长。细菌冰核蛋白 (INP) 通常很大并在细胞膜中形成聚集体,而昆虫高活性抗冻蛋白 (AFP) 是可溶的且通常很小。实验表明,较大的冰结合蛋白及其聚集体在较高温度下使冰成核。然而,仍然缺乏对冰结合蛋白的大小和聚集如何决定蛋白质使冰成核的温度 Thet 的定量理解。在这里,我们使用分子模拟和成核理论来解决这个问题。模拟表明,2.5 nm 长的抗冻蛋白 TmAFP 在高于均相成核温度 2 ± 1 °C 时使冰成核,与最近的实验非常吻合。我们预测向 TmAFP 添加冰结合环会增加 Thet,但不足以与细菌 INP 竞争效率。我们实施了一个准确的程序来使用经典成核理论确定有限尺寸表面的 Thet,并且在针对分子模拟中蛋白质的 Thet 验证理论后,我们使用它来预测 Ps 的 INP 的 Thet。丁香作为聚集体中蛋白质长度和数量的函数。我们得出结论,最多 34 个 INP 的组件已经达到了该细菌的 Thet = -2 °C 特征。有趣的是,我们发现 Thet 是聚集体中蛋白质之间距离的强烈变化的非单调函数。这表明,要达到最大的冷冻效率,细菌必须发挥精巧、
更新日期:2019-04-12
中文翻译:

冰结合蛋白的大小和聚集如何控制它们的冰成核效率
在低温下茁壮成长的生物会产生冰结合蛋白来控制冰的成核和生长。细菌冰核蛋白 (INP) 通常很大并在细胞膜中形成聚集体,而昆虫高活性抗冻蛋白 (AFP) 是可溶的且通常很小。实验表明,较大的冰结合蛋白及其聚集体在较高温度下使冰成核。然而,仍然缺乏对冰结合蛋白的大小和聚集如何决定蛋白质使冰成核的温度 Thet 的定量理解。在这里,我们使用分子模拟和成核理论来解决这个问题。模拟表明,2.5 nm 长的抗冻蛋白 TmAFP 在高于均相成核温度 2 ± 1 °C 时使冰成核,与最近的实验非常吻合。我们预测向 TmAFP 添加冰结合环会增加 Thet,但不足以与细菌 INP 竞争效率。我们实施了一个准确的程序来使用经典成核理论确定有限尺寸表面的 Thet,并且在针对分子模拟中蛋白质的 Thet 验证理论后,我们使用它来预测 Ps 的 INP 的 Thet。丁香作为聚集体中蛋白质长度和数量的函数。我们得出结论,最多 34 个 INP 的组件已经达到了该细菌的 Thet = -2 °C 特征。有趣的是,我们发现 Thet 是聚集体中蛋白质之间距离的强烈变化的非单调函数。这表明,要达到最大的冷冻效率,细菌必须发挥精巧、


















































 京公网安备 11010802027423号
京公网安备 11010802027423号