当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Origins of Stereoselectivity in Mannich Reactions Catalyzed by Chiral Vicinal Diamines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1021/acs.joc.8b00037 Shuming Chen 1 , K. N. Houk 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-02-13 00:00:00 , DOI: 10.1021/acs.joc.8b00037 Shuming Chen 1 , K. N. Houk 1
Affiliation
|
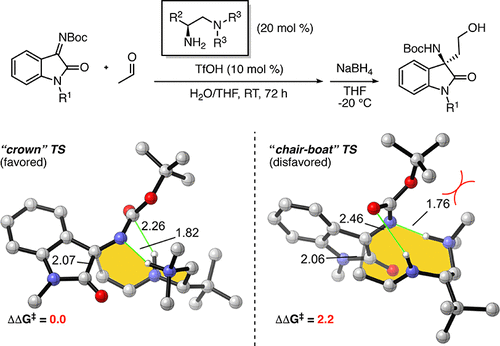
The origins of the enantio- and diastereoselectivities in the Mannich reactions between aldehydes and ketimines catalyzed by chiral vicinal diamines have been determined by density functional theory calculations and distortion–interaction analysis. Computational results indicate a strong energetic preference for hydrogen-bonded nine-membered cyclic transition states. The favored transition states involve eight heavy atoms in the crown (chair–chair) conformation using the nomenclature of the analogous cyclic hydrocarbons. Energetic discrimination in the chirality-imparting step arises from pseudogauche-butane-type interactions in the disfavored transition states, as well as steric clashes between the N-Boc protecting group and the ammonium N-substituents.
中文翻译:

手性邻二胺催化曼尼希反应中立体选择性的起源
通过手性邻位二胺催化醛和酮亚胺之间的曼尼希反应中对映和非对映选择性的起源已通过密度泛函理论计算和畸变-相互作用分析确定。计算结果表明,氢键键合的九元环状过渡态具有强烈的能量偏好。优先的过渡态使用类似的环状烃的命名法,在冠(椅-椅)构象中包含八个重原子。在手性赋予步骤中的高能辨别是由于在不利的过渡态下的假高脂-丁烷型相互作用,以及N- Boc保护基团和N-铵取代基之间的空间碰撞引起的。
更新日期:2018-02-13
中文翻译:

手性邻二胺催化曼尼希反应中立体选择性的起源
通过手性邻位二胺催化醛和酮亚胺之间的曼尼希反应中对映和非对映选择性的起源已通过密度泛函理论计算和畸变-相互作用分析确定。计算结果表明,氢键键合的九元环状过渡态具有强烈的能量偏好。优先的过渡态使用类似的环状烃的命名法,在冠(椅-椅)构象中包含八个重原子。在手性赋予步骤中的高能辨别是由于在不利的过渡态下的假高脂-丁烷型相互作用,以及N- Boc保护基团和N-铵取代基之间的空间碰撞引起的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号