Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cascade Reaction of 1,1-Enediamines with 2-Benzylidene-1H-indene-1,3(2H)-diones: Selective Synthesis of Indenodihydropyridine and Indenopyridine Compounds.
ACS Omega ( IF 3.7 ) Pub Date : 2019-04-11 , DOI: 10.1021/acsomega.9b00407 Qin Luo 1 , Rong Huang 1 , Qiang Xiao 1 , Ling-Bin Kong 1 , Jun Lin 1 , Sheng-Jiao Yan 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-04-11 , DOI: 10.1021/acsomega.9b00407 Qin Luo 1 , Rong Huang 1 , Qiang Xiao 1 , Ling-Bin Kong 1 , Jun Lin 1 , Sheng-Jiao Yan 1
Affiliation
|
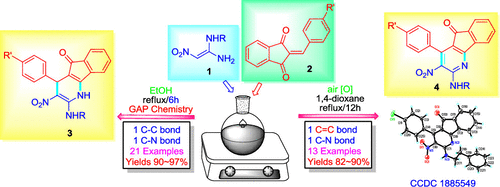
A concise and environmentally friendly route for the synthesis of diverse indenodihydropyridines (3) via a cascade reaction of 1,1-eneamines (1) with benzylidene-1H-indene-1,3(2H)-diones (BIDs) (2) in ethanol media was developed. The targeted compounds were efficiently obtained by only filtration without any further post-treatment. In the one-step cascade reaction, C-C and C-N bonds were constructed. In addition, when 1,4-dioxane was used as a solvent and the mixture of 1,1-eneamines (1) was refluxed with benzylidene-1H-indene-1,3(2H)-diones (BIDs) (2) for about 12 h, indenopyridine compounds (4) were produced. Two kinds of indenopyridine derivatives 3-4 resulted from alternative solvents and temperatures. The reaction had the following features: mild temperature, atom economy, high yields, and potential biological activity of the product.
中文翻译:

1,1-烯二胺与2-苄基-1H-茚-1,3(2H)-二酮的级联反应:茚并二氢吡啶和茚并吡啶化合物的选择性合成。
通过1,1-烯胺(1)与亚苄基-1H-茚-1,3(2H)-二酮(BID)(2)的级联反应合成各种茚并二氢吡啶(3)的简捷环保方法开发了乙醇培养基。仅通过过滤就可以有效地获得目标化合物,而无需任何进一步的后处理。在一步式级联反应中,构建了CC和CN键。此外,当使用1,4-二恶烷作为溶剂,并将1,1-烯胺(1)的混合物与亚苄基-1H-茚-1,3(2H)-二酮(BID)(2)回流时,大约12小时,产生茚并吡啶化合物(4)。两种茚并吡啶衍生物3-4是由不同的溶剂和温度引起的。该反应具有以下特征:温和的温度,原子经济性,高产率和产物的潜在生物活性。
更新日期:2019-04-11
中文翻译:

1,1-烯二胺与2-苄基-1H-茚-1,3(2H)-二酮的级联反应:茚并二氢吡啶和茚并吡啶化合物的选择性合成。
通过1,1-烯胺(1)与亚苄基-1H-茚-1,3(2H)-二酮(BID)(2)的级联反应合成各种茚并二氢吡啶(3)的简捷环保方法开发了乙醇培养基。仅通过过滤就可以有效地获得目标化合物,而无需任何进一步的后处理。在一步式级联反应中,构建了CC和CN键。此外,当使用1,4-二恶烷作为溶剂,并将1,1-烯胺(1)的混合物与亚苄基-1H-茚-1,3(2H)-二酮(BID)(2)回流时,大约12小时,产生茚并吡啶化合物(4)。两种茚并吡啶衍生物3-4是由不同的溶剂和温度引起的。该反应具有以下特征:温和的温度,原子经济性,高产率和产物的潜在生物活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号