当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing ion binding in the selectivity filter of the KcsA potassium channel
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-11 , DOI: 10.1021/jacs.9b01092 Cédric Eichmann 1 , Lukas Frey 1 , Innokentiy Maslennikov 2 , Roland Riek 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-04-11 , DOI: 10.1021/jacs.9b01092 Cédric Eichmann 1 , Lukas Frey 1 , Innokentiy Maslennikov 2 , Roland Riek 1
Affiliation
|
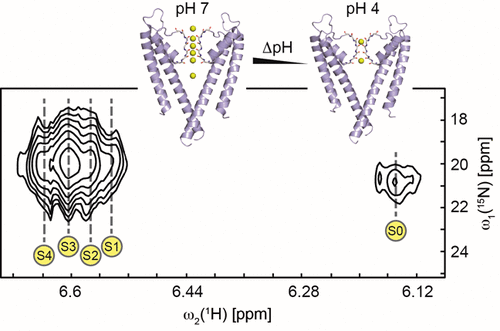
In potassium (K+) channels, permeation, selectivity, and gating at the selectivity filter are all governed by the thermodynamics and kinetics of the ion-protein interactions. Specific contacts between the carbonyl groups from the Thr-Val-Gly-Tyr-Gly signature filter sequence and the permeant ions generate four equidistant K+ binding sites, thereby defining the high ion selectivity and controlling the transport rate of K+ channels. Here, we used 15N-labeled ammonium (15NH4+) as a proxy for K+ to study ion interaction with the selectivity filter of the prototypical full-length K+ channel KcsA by solution state NMR spectroscopy in order to obtain detailed insights into the physicochemical basis of K+ gating. We found that in the closed inactive state of KcsA (at pH 7) four K+ binding sites are occupied over a wide range of 15NH4+ concentrations, while in intermediate closed-open conformations (at pH ∼6) the number and occupancy of K+ binding sites are reduced to two. However, in the presence of the scorpion toxin agitoxin II a total loss of 15NH4+ binding is observed. 15NH4+ titration studies allowed us to determine the dissociation constants of the four binding sites with values around 10 mM in the closed state of KcsA. Moreover, kinetic NMR experiments measured in the steady state equilibrium detected an off- and on-rate for 15NH4+ of ca. 102 s-1 and 103 s-1 between KcsA-bound 15NH4+ and the bulk. These findings reveal both the thermodynamics and kinetics of the ion binding sites and thus contribute to our understanding of the action of K+ channels.
中文翻译:

在 KcsA 钾通道的选择性过滤器中探测离子结合
在钾 (K+) 通道中,选择性过滤器的渗透、选择性和门控都受离子-蛋白质相互作用的热力学和动力学控制。来自 Thr-Val-Gly-Tyr-Gly 特征过滤器序列的羰基与渗透离子之间的特定接触产生四个等距的 K+ 结合位点,从而定义高离子选择性并控制 K+ 通道的传输速率。在这里,我们使用 15N 标记的铵 (15NH4+) 作为 K+ 的代理,通过溶液态核磁共振光谱研究离子与原型全长 K+ 通道 KcsA 的选择性过滤器的相互作用,以便详细了解 K+ 的物理化学基础门控。我们发现在 KcsA 的封闭非活性状态(pH 7)中,四个 K+ 结合位点被占据在广泛的 15NH4+ 浓度范围内,而在中间闭-开构象(pH 〜6)中,K+ 结合位点的数量和占有率减少到两个。然而,在蝎子毒素 agitoxin II 的存在下,观察到 15NH4+ 结合完全丧失。15NH4+ 滴定研究使我们能够确定四个结合位点的解离常数,在 KcsA 的闭合状态下,其值约为 10 mM。此外,在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。在蝎子毒素 agitoxin II 的存在下,观察到 15NH4+ 结合完全丧失。15NH4+ 滴定研究使我们能够确定四个结合位点的解离常数,在 KcsA 的闭合状态下,其值约为 10 mM。此外,在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。在蝎子毒素 agitoxin II 的存在下,观察到 15NH4+ 结合完全丧失。15NH4+ 滴定研究使我们能够确定四个结合位点的解离常数,在 KcsA 的闭合状态下,其值约为 10 mM。此外,在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。
更新日期:2019-04-11
中文翻译:

在 KcsA 钾通道的选择性过滤器中探测离子结合
在钾 (K+) 通道中,选择性过滤器的渗透、选择性和门控都受离子-蛋白质相互作用的热力学和动力学控制。来自 Thr-Val-Gly-Tyr-Gly 特征过滤器序列的羰基与渗透离子之间的特定接触产生四个等距的 K+ 结合位点,从而定义高离子选择性并控制 K+ 通道的传输速率。在这里,我们使用 15N 标记的铵 (15NH4+) 作为 K+ 的代理,通过溶液态核磁共振光谱研究离子与原型全长 K+ 通道 KcsA 的选择性过滤器的相互作用,以便详细了解 K+ 的物理化学基础门控。我们发现在 KcsA 的封闭非活性状态(pH 7)中,四个 K+ 结合位点被占据在广泛的 15NH4+ 浓度范围内,而在中间闭-开构象(pH 〜6)中,K+ 结合位点的数量和占有率减少到两个。然而,在蝎子毒素 agitoxin II 的存在下,观察到 15NH4+ 结合完全丧失。15NH4+ 滴定研究使我们能够确定四个结合位点的解离常数,在 KcsA 的闭合状态下,其值约为 10 mM。此外,在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。在蝎子毒素 agitoxin II 的存在下,观察到 15NH4+ 结合完全丧失。15NH4+ 滴定研究使我们能够确定四个结合位点的解离常数,在 KcsA 的闭合状态下,其值约为 10 mM。此外,在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。在蝎子毒素 agitoxin II 的存在下,观察到 15NH4+ 结合完全丧失。15NH4+ 滴定研究使我们能够确定四个结合位点的解离常数,在 KcsA 的闭合状态下,其值约为 10 mM。此外,在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。在稳态平衡中测量的动力学 NMR 实验检测到约 15NH4+ 的断开和接通速率。102 s-1 和 103 s-1 在 KcsA 结合的 15NH4+ 和主体之间。这些发现揭示了离子结合位点的热力学和动力学,从而有助于我们理解 K+ 通道的作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号