Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2019-04-10 , DOI: 10.1016/j.molliq.2019.04.047 Jinlong Li , Hong Zhu , Changjun Peng , Honglai Liu
|
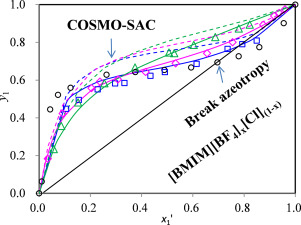
The effects of ionic liquid mixtures on the phase behavior of azeotropic mixture of acetonitrile + water were presented through the experimental determinations of isobaric vapor-liquid equilibrium for acetonitrile + water + 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) and acetonitrile + water + 1-butyl-3-methylimidazolium chloride ([Bmim][Cl]) + [BMIM][BF4] at atmospheric pressure (101.3 kPa). The mole ratios of [BMIM][Cl] to [BMIM][BF4] in the mixing ILs were fixed at 2:1, 1:1 and 1:2, respectively. The results showed that [BMIM][BF4] was ineffective in breaking the azeotropy of acetonitrile and water, which whereas could be broken when [BMIM][Cl] reached a certain concentration in the mixing ILs. VLE data of ternary and quaternary mixtures were satisfactorily correlated and predicted with NRTL model and COSMO-SAC could give a reasonable prediction of VLE for the investigated mixtures. Additionally, the analysis of interactions among different molecules showed that the interactions between [BMIM][Cl] and water are strong while ones between [BMIM][BF4] and water weak.
中文翻译:

[BMIM] [Cl]和[BMIM] [BF 4 ]的二元离子液体混合物对大气压下乙腈+水的等压汽液平衡的影响
通过实验确定乙腈+水+ 1-丁基-3-甲基咪唑四氟硼酸酯([BMIM] [BF 4 ] )和乙腈+水+ 1-丁基-3-甲基咪唑鎓氯化物([Bmim] [Cl])+ [BMIM] [BF 4 ]在大气压(101.3 kPa)下进行。混合IL中的[BMIM] [Cl]与[BMIM] [BF 4 ]的摩尔比分别固定为2∶1、1∶1和1∶2。结果表明,[BMIM] [BF 4]不能有效地破坏乙腈和水的共沸性,而当[BMIM] [Cl]在混合离子液体中达到一定浓度时,它可能被破坏。用NRTL模型对三元和四元混合物的VLE数据进行了令人满意的关联和预测,而COSMO-SAC可以为所研究的混合物提供合理的VLE预测。另外,对不同分子间相互作用的分析表明,[BMIM] [Cl]与水之间的相互作用较强,而[BMIM] [BF 4 ]与水之间的相互作用较弱。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号