Nature Communications ( IF 14.7 ) Pub Date : 2019-04-10 , DOI: 10.1038/s41467-019-09717-6 Scott Gladstein 1 , Luay M Almassalha 1 , Lusik Cherkezyan 1 , John E Chandler 1 , Adam Eshein 1 , Aya Eid 1 , Di Zhang 1 , Wenli Wu 1 , Greta M Bauer 1 , Andrew D Stephens 2 , Simona Morochnik 1 , Hariharan Subramanian 1, 3 , John F Marko 2, 4, 5 , Guillermo A Ameer 1, 3, 5 , Igal Szleifer 1, 3, 5 , Vadim Backman 1, 3, 5
|
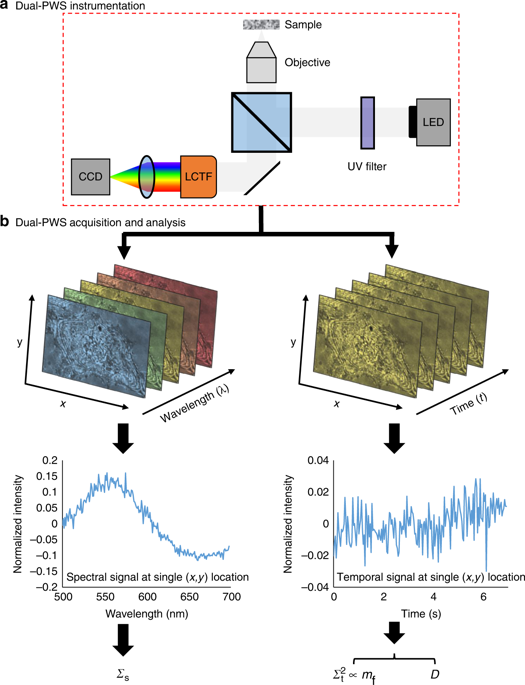
Understanding the relationship between intracellular motion and macromolecular structure remains a challenge in biology. Macromolecular structures are assembled from numerous molecules, some of which cannot be labeled. Most techniques to study motion require potentially cytotoxic dyes or transfection, which can alter cellular behavior and are susceptible to photobleaching. Here we present a multimodal label-free imaging platform for measuring intracellular structure and macromolecular dynamics in living cells with a sensitivity to macromolecular structure as small as 20 nm and millisecond temporal resolution. We develop and validate a theory for temporal measurements of light interference. In vitro, we study how higher-order chromatin structure and dynamics change during cell differentiation and ultraviolet (UV) light irradiation. Finally, we discover cellular paroxysms, a near-instantaneous burst of macromolecular motion that occurs during UV induced cell death. With nanoscale sensitive, millisecond resolved capabilities, this platform could address critical questions about macromolecular behavior in live cells.
中文翻译:

基于多模态干涉的纳米级结构和大分子运动成像揭示了紫外线诱导的细胞发作。
了解细胞内运动与大分子结构之间的关系仍然是生物学中的一个挑战。大分子结构由众多分子组装而成,其中一些分子无法被标记。大多数研究运动的技术都需要潜在的细胞毒性染料或转染,这可能会改变细胞行为并且容易受到光漂白的影响。在这里,我们提出了一种多模态无标记成像平台,用于测量活细胞的细胞内结构和大分子动力学,其对大分子结构的敏感性小至 20 nm 和毫秒时间分辨率。我们开发并验证了光干涉时间测量的理论。在体外,我们研究细胞分化和紫外线 (UV) 照射过程中高阶染色质结构和动力学如何变化。最后,我们发现了细胞阵发性,即紫外线诱导的细胞死亡过程中发生的近乎瞬时的大分子运动爆发。凭借纳米级敏感、毫秒级分辨能力,该平台可以解决有关活细胞中大分子行为的关键问题。































 京公网安备 11010802027423号
京公网安备 11010802027423号