当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DNA Adduct-Directed Synthetic Nucleosides.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-04-09 00:00:00 , DOI: 10.1021/acs.accounts.9b00054 Michael H Räz 1 , Claudia M N Aloisi 1 , Hailey L Gahlon 1 , Shana J Sturla 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-04-09 00:00:00 , DOI: 10.1021/acs.accounts.9b00054 Michael H Räz 1 , Claudia M N Aloisi 1 , Hailey L Gahlon 1 , Shana J Sturla 1
Affiliation
|
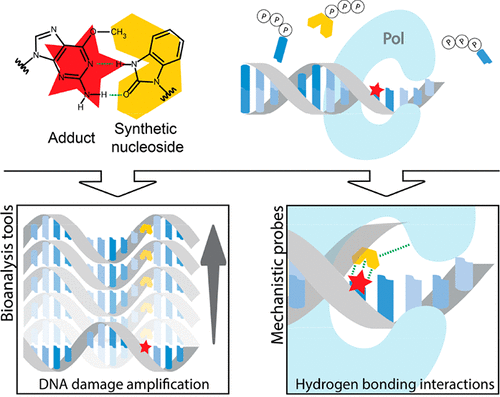
Chemical damage to DNA is a key initiator of adverse biological consequences due to disruption of the faithful reading of the genetic code. For example, O6-alkylguanine (O6-alkylG) DNA adducts are strongly miscoding during DNA replication when the damaged nucleobase is a template for polymerase-mediated translesion DNA synthesis. Thus, mutations derived from O6-alkylG adducts can have severe adverse effects on protein translation and function and are an early event in the initiation of carcinogenesis. However, the low abundance of these adducts places significant limitations on our ability to relate their presence and biological influences with resultant mutations or disease risk. As a consequence, there is a critical need for novel tools to detect and study the biological role of alkylation adducts. Incorporating DNA bases with altered structures that are derived synthetically is a strategy that has been used widely to interrogate biological processes involving DNA. Such synthetic nucleosides have contributed to our understanding of DNA structure, DNA polymerase (Pol) and repair enzyme function, and to the expansion of the genetic alphabet. This Account describes our efforts toward creating and applying synthetic nucleosides directed at DNA adducts. We synthesized a variety of nucleosides with altered base structures that complement the altered hydrogen bonding capacity and hydrophilicity of O6-alkylG adducts. The heterocyclic perimidinone-derived nucleoside Per was the first of such adduct-directed synthetic nucleosides; it specifically stabilized O6-benzylguanine (O6-BnG) in a DNA duplex. Structural variants of Per were used to determine hydrogen bonding and base-stacking contributions to DNA duplex stability in templates containing O6-BnG as well as O6-methylguanine (O6-MeG) adducts. We created synthetic probes able to stabilize damaged over undamaged templates and established how altered hydrogen bonding or base-stacking properties impact DNA duplex stability as a function of adduct structures. This knowledge was then applied to devise a hybridization-based detection strategy involving gold nanoparticles that distinguish damaged from undamaged DNA by colorimetric changes. Furthermore, synthetic nucleosides were used as mechanistic tools to understand chemical determinants such as hydrogen bonding, π-stacking, and size and shape deviations that impact the efficiency and fidelity of DNA adduct bypass by DNA Pols. Finally, we reported the first example of amplifying alkylated DNA, accomplished by combining an engineered polymerase and synthetic triphosphate for which incorporation is templated by a DNA adduct. The presence of the synthetic nucleoside in amplicons could serve as a marker for the presence and location of DNA damage at low levels in DNA strands. Adduct-directed synthetic nucleosides have opened new concepts to interrogate the levels, locations, and biological influences of DNA alkylation.
中文翻译:

DNA加合物定向合成核苷。
由于对遗传密码的忠实阅读的破坏,对DNA的化学损害是造成不良生物学后果的关键引发因素。例如,当受损的核碱基是聚合酶介导的转移性DNA合成的模板时,O 6烷基鸟嘌呤(O 6烷基G)DNA加合物在DNA复制过程中发生严重错误编码。因此,源自O 6的突变-烷基G加合物可能对蛋白质的翻译和功能产生严重的不利影响,并且是致癌作用开始的早期事件。但是,这些加合物的丰度很低,极大地限制了我们将它们的存在和生物学影响与所导致的突变或疾病风险相关联的能力。结果,迫切需要检测和研究烷基化加合物的生物学作用的新颖工具。将具有合成衍生的改变的结构的DNA碱基掺入是一种已被广泛用于询问涉及DNA的生物学过程的策略。此类合成核苷有助于我们对DNA结构,DNA聚合酶(Pol)和修复酶功能的理解,并有助于遗传字母的扩展。该说明描述了我们在创建和应用针对DNA加合物的合成核苷方面所做的努力。我们合成了具有改变的碱基结构的各种核苷,这些核苷补充了改变的氢键合能力和亲水性。O 6-烷基G加合物。杂环派米定酮衍生的核苷Per是这类加合物定向的合成核苷中的第一个。它可以特异性地稳定DNA双链体中的O 6-苄基鸟嘌呤(O 6 -BnG)。Per的结构变体用于确定包含O 6 -BnG和O 6-甲基鸟嘌呤(O 6-MeG)加合物。我们创建了能够稳定未损坏模板上的损坏的合成探针,并确定了氢键或碱基堆积特性的改变如何影响加合物结构对DNA双链体稳定性的影响。然后,将这些知识应用于设计基于杂交的检测策略,该策略涉及金纳米颗粒,该纳米颗粒通过比色变化将损坏的DNA与未损坏的DNA进行区分。此外,合成核苷还用作机理工具来了解化学决定因素,例如氢键,π堆积以及影响DNA Pols绕过DNA加合物的效率和保真度的大小和形状偏差。最后,我们报道了扩增烷基化DNA的第一个实例,该实例是通过将工程改造的聚合酶和合成的三磷酸酯结合在一起而实现的,合成的三磷酸酯是通过DNA加合物将掺入模板化的。扩增子中合成核苷的存在可以作为DNA链中低水平的DNA损伤的存在和定位的标记。针对加合物的合成核苷已经开辟了新的概念,可以研究DNA烷基化的水平,位置和生物学影响。
更新日期:2019-04-09
中文翻译:

DNA加合物定向合成核苷。
由于对遗传密码的忠实阅读的破坏,对DNA的化学损害是造成不良生物学后果的关键引发因素。例如,当受损的核碱基是聚合酶介导的转移性DNA合成的模板时,O 6烷基鸟嘌呤(O 6烷基G)DNA加合物在DNA复制过程中发生严重错误编码。因此,源自O 6的突变-烷基G加合物可能对蛋白质的翻译和功能产生严重的不利影响,并且是致癌作用开始的早期事件。但是,这些加合物的丰度很低,极大地限制了我们将它们的存在和生物学影响与所导致的突变或疾病风险相关联的能力。结果,迫切需要检测和研究烷基化加合物的生物学作用的新颖工具。将具有合成衍生的改变的结构的DNA碱基掺入是一种已被广泛用于询问涉及DNA的生物学过程的策略。此类合成核苷有助于我们对DNA结构,DNA聚合酶(Pol)和修复酶功能的理解,并有助于遗传字母的扩展。该说明描述了我们在创建和应用针对DNA加合物的合成核苷方面所做的努力。我们合成了具有改变的碱基结构的各种核苷,这些核苷补充了改变的氢键合能力和亲水性。O 6-烷基G加合物。杂环派米定酮衍生的核苷Per是这类加合物定向的合成核苷中的第一个。它可以特异性地稳定DNA双链体中的O 6-苄基鸟嘌呤(O 6 -BnG)。Per的结构变体用于确定包含O 6 -BnG和O 6-甲基鸟嘌呤(O 6-MeG)加合物。我们创建了能够稳定未损坏模板上的损坏的合成探针,并确定了氢键或碱基堆积特性的改变如何影响加合物结构对DNA双链体稳定性的影响。然后,将这些知识应用于设计基于杂交的检测策略,该策略涉及金纳米颗粒,该纳米颗粒通过比色变化将损坏的DNA与未损坏的DNA进行区分。此外,合成核苷还用作机理工具来了解化学决定因素,例如氢键,π堆积以及影响DNA Pols绕过DNA加合物的效率和保真度的大小和形状偏差。最后,我们报道了扩增烷基化DNA的第一个实例,该实例是通过将工程改造的聚合酶和合成的三磷酸酯结合在一起而实现的,合成的三磷酸酯是通过DNA加合物将掺入模板化的。扩增子中合成核苷的存在可以作为DNA链中低水平的DNA损伤的存在和定位的标记。针对加合物的合成核苷已经开辟了新的概念,可以研究DNA烷基化的水平,位置和生物学影响。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号