Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalase-Integrated Hyaluronic Acid as Nanocarriers for Enhanced Photodynamic Therapy in Solid Tumor.
ACS Nano ( IF 15.8 ) Pub Date : 2019-04-09 00:00:00 , DOI: 10.1021/acsnano.9b01087 Soo Zeng Fiona Phua 1 , Guangbao Yang 1 , Wei Qi Lim 1, 2 , Apoorva Verma 3 , Hongzhong Chen 1 , Thirumaran Thanabalu 3 , Yanli Zhao 1
ACS Nano ( IF 15.8 ) Pub Date : 2019-04-09 00:00:00 , DOI: 10.1021/acsnano.9b01087 Soo Zeng Fiona Phua 1 , Guangbao Yang 1 , Wei Qi Lim 1, 2 , Apoorva Verma 3 , Hongzhong Chen 1 , Thirumaran Thanabalu 3 , Yanli Zhao 1
Affiliation
|
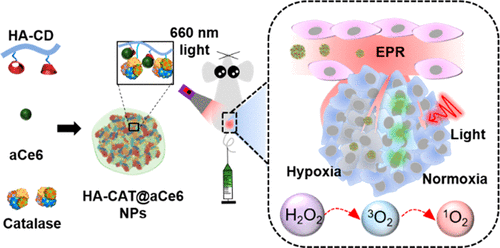
Photodynamic therapy (PDT) as a treatment method has many advantages such as minimal invasiveness, repeatable dosage, and low systemic toxicity. Issues with conventional PDT agents include the limited availability of endogenous oxygen and difficulty in accumulation at the tumor site, which has hindered the successful treatment of tumors. Herein, we developed catalase-encapsulated hyaluronic-acid-based nanoparticles loaded with adamantane-modified photosensitizer for enhanced PDT of solid tumors. Chlorin e6 (Ce6) as the photosensitizer was modified with adamantane to yield adamantane-modified Ce6 (aCe6). The obtained nanosystem ([email protected]) could target overly expressed CD44 receptors on cancer cells, supplying oxygen by converting endogenous hydrogen peroxide (H2O2) to oxygen, and improving PDT efficacy upon light irradiation. [email protected] nanoparticles showed high colloidal stability and monodispersity in aqueous solution. The uptake and targeting property of [email protected] were demonstrated by confocal microscopy and flow cytometry in the MDA-MB-231 cell line possessing overly expressed CD44 receptors. The encapsulated catalase was able to decompose the endogenous H2O2 to generate O2in situ for relieving hypoxia in cells incubated under hypoxic conditions. Cell viability assays indicated that [email protected] possessed minimal cytotoxicity in the dark, while presenting high cellular toxicity under 660 nm light irradiation at normoxic conditions. As a result of the catalase capability in relieving hypoxia, [email protected] also exhibited high cellular cytotoxicity under hypoxic condition. In vivo experiments revealed selective tumor accumulation of [email protected] in MDA-MB-231 tumor bearing nude mice. Significant tumor regression was observed after intravenous injection of [email protected] under light irradiation in comparison to the control system without loading catalase. Thus, [email protected] demonstrated a great potential in overcoming hypoxia for targeted PDT.
中文翻译:

过氧化氢酶整合的透明质酸作为纳米载体,可增强实体瘤的光动力治疗。
光动力疗法(PDT)作为一种治疗方法具有许多优势,例如最小的侵入性,可重复的剂量和较低的全身毒性。常规PDT剂的问题包括内源性氧的有限供应和在肿瘤部位积累的困难,这阻碍了肿瘤的成功治疗。在本文中,我们开发了负载过金刚烷修饰的光敏剂的过氧化氢酶包封的透明质酸基纳米颗粒,用于增强实体瘤的PDT。用金刚烷修饰作为光敏剂的氯霉素e6(Ce6),得到金刚烷修饰的Ce6(aCe6)。所获得的纳米系统([受电子邮件保护])可以靶向癌细胞上过度表达的CD44受体,通过转化内源性过氧化氢(H 2 O 2)释放氧气,并提高光照射后的PDT功效。[受电子邮件保护]纳米粒子在水溶液中显示出高的胶体稳定性和单分散性。通过共聚焦显微镜和流式细胞术在具有过度表达的CD44受体的MDA-MB-231细胞系中证明了[电子邮件保护的]的摄取和靶向特性。包封的过氧化氢酶能够分解内源性H 2 O 2以原位产生O 2用于缓解在缺氧条件下培养的细胞中的缺氧。细胞活力测定表明,[电子邮件保护的]在黑暗中具有最小的细胞毒性,而在常氧条件下在660 nm光照射下表现出很高的细胞毒性。由于过氧化氢酶具有缓解缺氧的能力,在缺氧条件下,[电子邮件保护的]也表现出很高的细胞毒性。体内实验显示,在带有MDA-MB-231荷瘤裸鼠的肿瘤中,[电子邮件保护的]选择性肿瘤蓄积。与不加过氧化氢酶的对照系统相比,在光照射下静脉注射[电子邮件保护的]后观察到明显的肿瘤消退。因此,[电子邮件保护]在克服针对性PDT的低氧方面显示出巨大的潜力。
更新日期:2019-04-09
中文翻译:

过氧化氢酶整合的透明质酸作为纳米载体,可增强实体瘤的光动力治疗。
光动力疗法(PDT)作为一种治疗方法具有许多优势,例如最小的侵入性,可重复的剂量和较低的全身毒性。常规PDT剂的问题包括内源性氧的有限供应和在肿瘤部位积累的困难,这阻碍了肿瘤的成功治疗。在本文中,我们开发了负载过金刚烷修饰的光敏剂的过氧化氢酶包封的透明质酸基纳米颗粒,用于增强实体瘤的PDT。用金刚烷修饰作为光敏剂的氯霉素e6(Ce6),得到金刚烷修饰的Ce6(aCe6)。所获得的纳米系统([受电子邮件保护])可以靶向癌细胞上过度表达的CD44受体,通过转化内源性过氧化氢(H 2 O 2)释放氧气,并提高光照射后的PDT功效。[受电子邮件保护]纳米粒子在水溶液中显示出高的胶体稳定性和单分散性。通过共聚焦显微镜和流式细胞术在具有过度表达的CD44受体的MDA-MB-231细胞系中证明了[电子邮件保护的]的摄取和靶向特性。包封的过氧化氢酶能够分解内源性H 2 O 2以原位产生O 2用于缓解在缺氧条件下培养的细胞中的缺氧。细胞活力测定表明,[电子邮件保护的]在黑暗中具有最小的细胞毒性,而在常氧条件下在660 nm光照射下表现出很高的细胞毒性。由于过氧化氢酶具有缓解缺氧的能力,在缺氧条件下,[电子邮件保护的]也表现出很高的细胞毒性。体内实验显示,在带有MDA-MB-231荷瘤裸鼠的肿瘤中,[电子邮件保护的]选择性肿瘤蓄积。与不加过氧化氢酶的对照系统相比,在光照射下静脉注射[电子邮件保护的]后观察到明显的肿瘤消退。因此,[电子邮件保护]在克服针对性PDT的低氧方面显示出巨大的潜力。















































 京公网安备 11010802027423号
京公网安备 11010802027423号