当前位置:
X-MOL 学术
›
Cancer Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein arginine methyltransferase 5: A novel therapeutic target for triple-negative breast cancers.
Cancer Medicine ( IF 2.9 ) Pub Date : 2019-04-08 , DOI: 10.1002/cam4.2114 Mathilde Vinet 1, 2 , Samyuktha Suresh 1, 2 , Virginie Maire 1, 2 , Clarisse Monchecourt 1, 2 , Fariba Némati 1, 3 , Laetitia Lesage 4 , Fabienne Pierre 1, 2, 5 , Mengliang Ye 1, 2 , Auriane Lescure 1, 5 , Amélie Brisson 1, 2 , Didier Meseure 4 , André Nicolas 4 , Guillem Rigaill 6, 7 , Elisabetta Marangoni 1, 3 , Elaine Del Nery 1, 5 , Sergio Roman-Roman 1 , Thierry Dubois 1, 2
Cancer Medicine ( IF 2.9 ) Pub Date : 2019-04-08 , DOI: 10.1002/cam4.2114 Mathilde Vinet 1, 2 , Samyuktha Suresh 1, 2 , Virginie Maire 1, 2 , Clarisse Monchecourt 1, 2 , Fariba Némati 1, 3 , Laetitia Lesage 4 , Fabienne Pierre 1, 2, 5 , Mengliang Ye 1, 2 , Auriane Lescure 1, 5 , Amélie Brisson 1, 2 , Didier Meseure 4 , André Nicolas 4 , Guillem Rigaill 6, 7 , Elisabetta Marangoni 1, 3 , Elaine Del Nery 1, 5 , Sergio Roman-Roman 1 , Thierry Dubois 1, 2
Affiliation
|
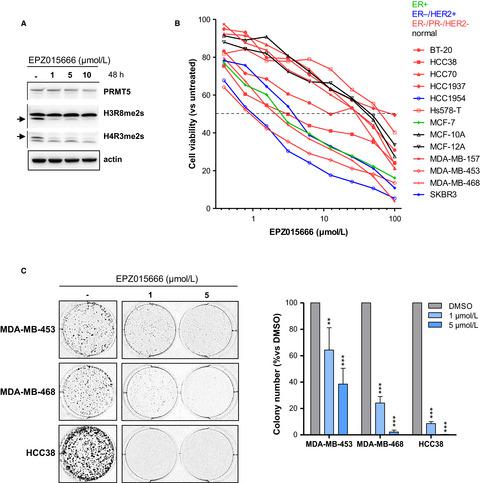
TNBC is a highly heterogeneous and aggressive breast cancer subtype associated with high relapse rates, and for which no targeted therapy yet exists. Protein arginine methyltransferase 5 (PRMT5), an enzyme which catalyzes the methylation of arginines on histone and non-histone proteins, has recently emerged as a putative target for cancer therapy. Potent and specific PRMT5 inhibitors have been developed, but the therapeutic efficacy of PRMT5 targeting in TNBC has not yet been demonstrated. Here, we examine the expression of PRMT5 in a human breast cancer cohort obtained from the Institut Curie, and evaluate the therapeutic potential of pharmacological inhibition of PRMT5 in TNBC. We find that PRMT5 mRNA and protein are expressed at comparable levels in TNBC, luminal breast tumors, and healthy mammary tissues. However, immunohistochemistry analyses reveal that PRMT5 is differentially localized in TNBC compared to other breast cancer subtypes and to normal breast tissues. PRMT5 is heterogeneously expressed in TNBC and high PRMT5 expression correlates with poor prognosis within this breast cancer subtype. Using the small-molecule inhibitor EPZ015666, we show that PRMT5 inhibition impairs cell proliferation in a subset of TNBC cell lines. PRMT5 inhibition triggers apoptosis, regulates cell cycle progression and decreases mammosphere formation. Furthermore, EPZ015666 administration to a patient-derived xenograft model of TNBC significantly deters tumor progression. Finally, we reveal potentiation between EGFR and PRMT5 targeting, suggestive of a beneficial combination therapy. Our findings highlight a distinctive subcellular localization of PRMT5 in TNBC, and uphold PRMT5 targeting, alone or in combination, as a relevant treatment strategy for a subset of TNBC.
中文翻译:

蛋白质精氨酸甲基转移酶5:三阴性乳腺癌的新型治疗靶点。
TNBC是与高复发率相关的高度异质性和侵袭性乳腺癌亚型,目前尚无针对性的治疗方法。蛋白质精氨酸甲基转移酶5(PRMT5)是一种催化精氨酸在组蛋白和非组蛋白上甲基化的酶,最近已被证明是癌症治疗的靶标。已经开发了有效的和特异性的PRMT5抑制剂,但是尚未证明PRMT5靶向TNBC的治疗功效。在这里,我们检查了PRMT5在从居里研究所获得的人类乳腺癌队列中的表达,并评估了PRMT5在TNBC中的药理抑制作用。我们发现PRMT5 mRNA和蛋白在TNBC,管腔乳腺肿瘤和健康的乳腺组织中以可比较的水平表达。然而,免疫组织化学分析显示,与其他乳腺癌亚型和正常乳腺组织相比,PRMT5在TNBC中的定位存在差异。PRMT5在TNBC中异质表达,PRMT5高表达与该乳腺癌亚型的不良预后相关。使用小分子抑制剂EPZ015666,我们显示PRMT5抑制作用会损害TNBC细胞系子集中的细胞增殖。PRMT5抑制可触发细胞凋亡,调节细胞周期进程并减少乳球形成。此外,对患者来源的TNBC异种移植模型进行EPZ015666给药可显着阻止肿瘤进展。最后,我们揭示了靶向EGFR和PRMT5之间的增强作用,提示了一种有益的联合疗法。我们的发现突显了PRMT5在TNBC中的独特亚细胞定位,
更新日期:2019-05-16
中文翻译:

蛋白质精氨酸甲基转移酶5:三阴性乳腺癌的新型治疗靶点。
TNBC是与高复发率相关的高度异质性和侵袭性乳腺癌亚型,目前尚无针对性的治疗方法。蛋白质精氨酸甲基转移酶5(PRMT5)是一种催化精氨酸在组蛋白和非组蛋白上甲基化的酶,最近已被证明是癌症治疗的靶标。已经开发了有效的和特异性的PRMT5抑制剂,但是尚未证明PRMT5靶向TNBC的治疗功效。在这里,我们检查了PRMT5在从居里研究所获得的人类乳腺癌队列中的表达,并评估了PRMT5在TNBC中的药理抑制作用。我们发现PRMT5 mRNA和蛋白在TNBC,管腔乳腺肿瘤和健康的乳腺组织中以可比较的水平表达。然而,免疫组织化学分析显示,与其他乳腺癌亚型和正常乳腺组织相比,PRMT5在TNBC中的定位存在差异。PRMT5在TNBC中异质表达,PRMT5高表达与该乳腺癌亚型的不良预后相关。使用小分子抑制剂EPZ015666,我们显示PRMT5抑制作用会损害TNBC细胞系子集中的细胞增殖。PRMT5抑制可触发细胞凋亡,调节细胞周期进程并减少乳球形成。此外,对患者来源的TNBC异种移植模型进行EPZ015666给药可显着阻止肿瘤进展。最后,我们揭示了靶向EGFR和PRMT5之间的增强作用,提示了一种有益的联合疗法。我们的发现突显了PRMT5在TNBC中的独特亚细胞定位,































 京公网安备 11010802027423号
京公网安备 11010802027423号