当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Lysosome-Targeted Phosphine-Imine Half-Sandwich Iridium(III) Anticancer Complexes: Synthesis, Characterization, and Biological Activity
Organometallics ( IF 2.5 ) Pub Date : 2019-04-09 , DOI: 10.1021/acs.organomet.9b00080 Yuliang Yang 1 , Lihua Guo 1 , Zhenzhen Tian 1 , Xingxing Ge 1 , Yuteng Gong 1 , Hongmei Zheng 1 , Shaopeng Shi 1 , Zhe Liu 1
Organometallics ( IF 2.5 ) Pub Date : 2019-04-09 , DOI: 10.1021/acs.organomet.9b00080 Yuliang Yang 1 , Lihua Guo 1 , Zhenzhen Tian 1 , Xingxing Ge 1 , Yuteng Gong 1 , Hongmei Zheng 1 , Shaopeng Shi 1 , Zhe Liu 1
Affiliation
|
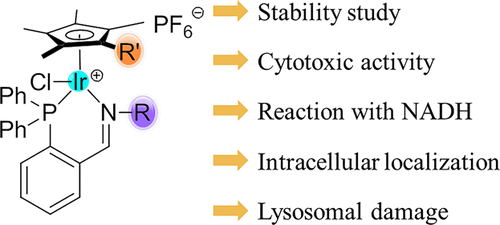
The synthesis, characterization, and catalytic ability of converting coenzyme NADH to NAD+ and the anticancer activity of half-sandwich iridium(III) complexes with general formula of [(η5-Cpx)Ir(P^N)Cl]PF6 (Cpx: Cp* or biphenyl Cpxbiph derivatives; P^N: various phosphine-imine ligands) were investigated. The crystal structure of the complex Ir4 showed a piano-stool geometry around the iridium(III) center. This type of iridium(III) complexes had sufficient stability in aqueous solution. Most of the complexes showed good anticancer activities toward A549 cancer cells, which were higher than the clinical drug cisplatin. In this series, complex Ir8 displayed the highest anticancer activity against A549 cells (IC50 = 4.7 μM), showing an approximately 4.5-fold more potent activity than cisplatin (IC50 = 21.30 μM). The structure–activity relationship study showed that the cytotoxicity of these complexes may be primarily attributed to the coordination between iridium(III) and the coordinating atoms, and the nature of the imine N-substituents may not be a major factor affecting cytotoxicity. Furthermore, this family of complexes causes cell death by cell stress, inducing apoptosis and necrosis, overproduction of reactive oxygen species, and disruption of the mitochondrial membrane potential. Most interestingly, the use of confocal microscopy provides insights into the microscopic mechanism that the typical complex Ir3 can penetrate into A549 cancer cells through a non-energy-dependent pathway and specifically distribute in lysosomes.
中文翻译:

溶酶体靶向的膦亚胺半三明治铱(III)抗癌复合物:合成,表征和生物活性。
的合成,表征,和辅酶催化NADH转化为NAD的能力+和半夹心铱(III)与[(η通式配合物的抗癌活性5 -Cp X)IR(P ^ N)CL] PF 6研究了(Cp x:Cp *或联苯Cp xbiph衍生物; P ^ N:各种膦亚胺配体)。复合物Ir4的晶体结构在铱(III)中心周围显示出钢琴凳的几何形状。这类铱(III)配合物在水溶液中具有足够的稳定性。大多数复合物对A549癌细胞显示出良好的抗癌活性,高于临床药物顺铂。在这个系列中,复杂的Ir8对A549细胞具有最高的抗癌活性(IC 50 = 4.7μM),显示出比顺铂(IC 50 = 21.30μM)强大约4.5倍的有效活性。结构-活性关系研究表明,这些配合物的细胞毒性可能主要归因于铱(III)与配位原子之间的配位,而亚胺N取代基的性质可能不是影响细胞毒性的主要因素。此外,该复合物家族通过细胞应激导致细胞死亡,诱导细胞凋亡和坏死,活性氧的过量产生以及线粒体膜电位的破坏。最有趣的是,共聚焦显微镜的使用为典型的复杂Ir3的微观机理提供了见解。 可通过非能量依赖性途径渗透到A549癌细胞中,并特异性地分布在溶酶体中。
更新日期:2019-04-09
中文翻译:

溶酶体靶向的膦亚胺半三明治铱(III)抗癌复合物:合成,表征和生物活性。
的合成,表征,和辅酶催化NADH转化为NAD的能力+和半夹心铱(III)与[(η通式配合物的抗癌活性5 -Cp X)IR(P ^ N)CL] PF 6研究了(Cp x:Cp *或联苯Cp xbiph衍生物; P ^ N:各种膦亚胺配体)。复合物Ir4的晶体结构在铱(III)中心周围显示出钢琴凳的几何形状。这类铱(III)配合物在水溶液中具有足够的稳定性。大多数复合物对A549癌细胞显示出良好的抗癌活性,高于临床药物顺铂。在这个系列中,复杂的Ir8对A549细胞具有最高的抗癌活性(IC 50 = 4.7μM),显示出比顺铂(IC 50 = 21.30μM)强大约4.5倍的有效活性。结构-活性关系研究表明,这些配合物的细胞毒性可能主要归因于铱(III)与配位原子之间的配位,而亚胺N取代基的性质可能不是影响细胞毒性的主要因素。此外,该复合物家族通过细胞应激导致细胞死亡,诱导细胞凋亡和坏死,活性氧的过量产生以及线粒体膜电位的破坏。最有趣的是,共聚焦显微镜的使用为典型的复杂Ir3的微观机理提供了见解。 可通过非能量依赖性途径渗透到A549癌细胞中,并特异性地分布在溶酶体中。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号