当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of Termicalcicolanone A via Organocatalysis and Regioselective Claisen Rearrangement
Organic Letters ( IF 4.9 ) Pub Date : 2019-04-08 00:00:00 , DOI: 10.1021/acs.orglett.9b00731 Saki Ito 1 , Taiki Kitamura 2 , Sundaram Arulmozhiraja 3, 4 , Kei Manabe 2 , Hiroaki Tokiwa 3, 4 , Yumiko Suzuki 1
Organic Letters ( IF 4.9 ) Pub Date : 2019-04-08 00:00:00 , DOI: 10.1021/acs.orglett.9b00731 Saki Ito 1 , Taiki Kitamura 2 , Sundaram Arulmozhiraja 3, 4 , Kei Manabe 2 , Hiroaki Tokiwa 3, 4 , Yumiko Suzuki 1
Affiliation
|
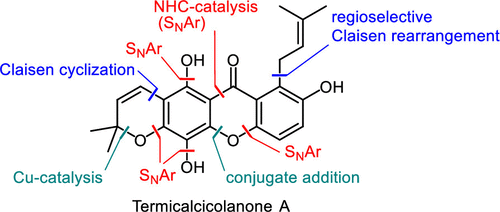
A total synthesis of an anticancer xanthone natural product termicalcicolanone A utilizing multiple nucleophilic aromatic substitutions and pericyclic reactions has been developed. The pyrano[3,2-b]xanthen-6-one scaffold was constructed via NHC-catalyzed aroylation to produce the benzophenone intermediate, Claisen cyclization to form the pyran ring, and intramolecular 1,4-addition to construct the xanthone framework. The prenyl group was introduced in the final stages of the synthesis through regioselective Claisen rearrangement. The synthesis has been achieved in 19 steps.
中文翻译:

通过有机催化和区域选择性克莱森重排完全合成TermicalcicolanoneA。
已经开发了利用多个亲核性芳族取代和周环反应的抗癌黄酮天然产物termicalcicolanone A的全合成。吡喃并[3,2 - b ]黄嘌呤-6-骨架是通过NHC催化的芳基化反应生成的二苯甲酮中间体,经克莱森环化形成吡喃环,并通过分子内的1,4-加成反应构建an吨酮骨架。通过区域选择性克莱森重排在合成的最后阶段引入异戊二烯基。合成已通过19个步骤完成。
更新日期:2019-04-08
中文翻译:

通过有机催化和区域选择性克莱森重排完全合成TermicalcicolanoneA。
已经开发了利用多个亲核性芳族取代和周环反应的抗癌黄酮天然产物termicalcicolanone A的全合成。吡喃并[3,2 - b ]黄嘌呤-6-骨架是通过NHC催化的芳基化反应生成的二苯甲酮中间体,经克莱森环化形成吡喃环,并通过分子内的1,4-加成反应构建an吨酮骨架。通过区域选择性克莱森重排在合成的最后阶段引入异戊二烯基。合成已通过19个步骤完成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号