Catalysis Today ( IF 5.2 ) Pub Date : 2019-04-09 , DOI: 10.1016/j.cattod.2019.04.027
Alejo Aguirre , Sebastián E. Collins
|
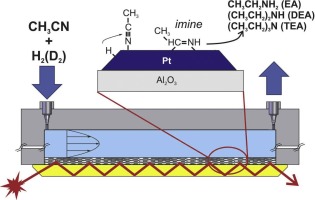
The mechanism of the acetonitrile hydrogenation to amines over a platinum/alumina catalyst was investigated by in situ infrared spectroscopy. The reaction was studied under realistic conditions -liquid phase using toluene as solvent- using a flow-through cell-microreactor in Attenuated Total Reflection (ATR) mode. Acetonitrile linearly chemisorbed on platinum sites is stepwise hydrogenated to form an imine surface intermediate (CH3CH = NH), which in turn is hydrogenated to ethylamine. Using deuterium, the sequential formation of CD and N
D bonds was observed. Then, imine intermediate can condense producing diethylamine and triethylamine, giving ammonia as a by-product. The temporal evolution of the IR signals was modeled using a proposed microkinetic mechanism and intrinsic kinetic constants were obtained under chemical control.
中文翻译:

ATR-FTIR对Pt / Al 2 O 3上液相乙腈加氢机理的认识
通过原位红外光谱研究了在铂/氧化铝催化剂上乙腈加氢成胺的机理。在现实条件下对反应进行了研究-使用甲苯作为溶剂的液相-使用流通池式微反应器以衰减全反射(ATR)模式进行研究。将线性化学吸附在铂位上的乙腈逐步氢化以形成亚胺表面中间体(CH 3 CH = NH),然后将其氢化成乙胺。使用氘,依次形成C D和N
观察到D键。然后,亚胺中间体可以缩合生成二乙胺和三乙胺,从而产生氨副产物。使用提出的微动力学机制对红外信号的时间演变进行建模,并在化学控制下获得内在动力学常数。































 京公网安备 11010802027423号
京公网安备 11010802027423号