当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Oxidation of Acetaldehyde to Acetic Acid on Pd–Au Bimetallic Model Catalysts
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-04-09 00:00:00 , DOI: 10.1021/acscatal.9b00079 Sungmin Han 1 , Kihyun Shin 1, 2 , Graeme Henkelman 1, 2 , C. Buddie Mullins 1, 3
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-04-09 00:00:00 , DOI: 10.1021/acscatal.9b00079 Sungmin Han 1 , Kihyun Shin 1, 2 , Graeme Henkelman 1, 2 , C. Buddie Mullins 1, 3
Affiliation
|
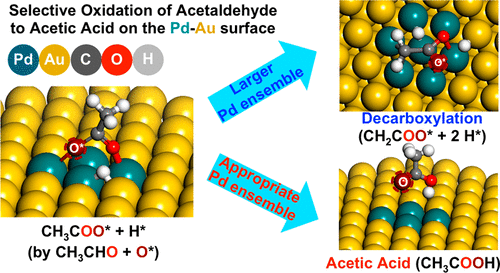
Acetic acid is a widely employed reactant in the chemical industry, and it is also used as a food ingredient. Here, we report a catalytic reaction pathway for the gas-phase selective oxidation of acetaldehyde to acetic acid on a Pd–Au(111) heterogeneous model catalyst. On an oxygen precovered Pd–Au surface, acetaldehyde is selectively oxidized to acetic acid from 250 to 340 K. Using FT-IR, the formation of acetate species is detected from 160 to 260 K on this surface, which is a widely known adsorbate derived from acetic acid on metal surfaces. With higher Pd coverages, the acetaldehyde is less selectively oxidized to acetic acid, and near 375 K, CO2, H2O, CH4, and H2 are evolved, evidence for the decarboxylation of acetate. In our density functional theory calculations, we confirm that the relative energy difference between the acetate state and the decarboxylated state decreases as Pd ensemble size increases.
中文翻译:

Pd-Au双金属模型催化剂上乙醛选择性氧化为乙酸
乙酸是化学工业中广泛使用的反应物,也被用作食品成分。在这里,我们报告了在Pd-Au(111)非均相模型催化剂上乙醛气相选择性氧化为乙酸的催化反应途径。在预先覆盖氧气的Pd-Au表面上,乙醛被选择性氧化为250至340 K的乙酸。使用FT-IR,检测到该表面160至260 K处乙酸盐的形成,这是一种众所周知的吸附物是从金属表面上的乙酸中提取的。在较高的Pd覆盖率下,乙醛的选择性氧化程度不高,接近375 K,CO 2,H 2 O,CH 4和H 2。不断进化,证明了乙酸盐脱羧的证据。在我们的密度泛函理论计算中,我们确认了乙酸态和脱羧态之间的相对能差随Pd团簇尺寸的增加而减小。
更新日期:2019-04-09
中文翻译:

Pd-Au双金属模型催化剂上乙醛选择性氧化为乙酸
乙酸是化学工业中广泛使用的反应物,也被用作食品成分。在这里,我们报告了在Pd-Au(111)非均相模型催化剂上乙醛气相选择性氧化为乙酸的催化反应途径。在预先覆盖氧气的Pd-Au表面上,乙醛被选择性氧化为250至340 K的乙酸。使用FT-IR,检测到该表面160至260 K处乙酸盐的形成,这是一种众所周知的吸附物是从金属表面上的乙酸中提取的。在较高的Pd覆盖率下,乙醛的选择性氧化程度不高,接近375 K,CO 2,H 2 O,CH 4和H 2。不断进化,证明了乙酸盐脱羧的证据。在我们的密度泛函理论计算中,我们确认了乙酸态和脱羧态之间的相对能差随Pd团簇尺寸的增加而减小。































 京公网安备 11010802027423号
京公网安备 11010802027423号