当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
General Platform for the Conversion of Isoxazol‐5‐ones to 3,5‐Disubstituted Isoxazoles via Nucleophilic Substitutions and Palladium Catalyzed Cross‐Coupling Strategies
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-05-09 , DOI: 10.1002/ejoc.201900187 Alessandra A. G. Fernandes 1 , Amanda F. da Silva 1 , Celso Y. Okada 1 , Vitor Suzukawa 1 , Rodrigo A. Cormanich 1 , Igor D. Jurberg 1
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2019-05-09 , DOI: 10.1002/ejoc.201900187 Alessandra A. G. Fernandes 1 , Amanda F. da Silva 1 , Celso Y. Okada 1 , Vitor Suzukawa 1 , Rodrigo A. Cormanich 1 , Igor D. Jurberg 1
Affiliation
|
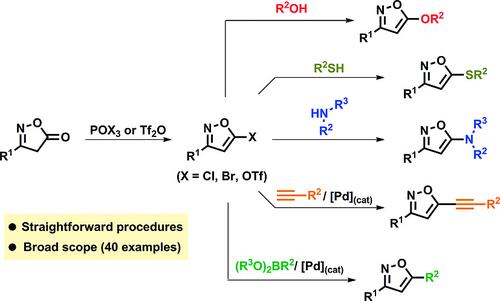
A general platform for the conversion of isoxazol‐5‐ones to 3,5‐disubstituted isoxazoles has been developed via a two‐step strategy. The first step leads to the formation of 5‐(pseudo)halogenated isoxazoles, while in the second, a variety of heteroalkyl‐, heteroaryl‐, alkyl‐, alkenyl‐, alkynyl‐ and aryl‐chains can be installed via nucleophilic substitutions or palladium catalyzed cross‐coupling reactions.

中文翻译:

通过亲核取代和钯催化的交叉偶联策略将异恶唑-5-酮转化为3,5-二取代的异恶唑的通用平台
通过两步策略,已经开发出了将异恶唑-5-酮转化为3,5-二取代异恶唑的通用平台。第一步导致5-(假)卤代异恶唑的形成,而第二步则可以通过亲核取代或钯安装各种杂烷基链,杂芳基链,烷基链,烯基链,炔基链和芳基链催化的交叉偶联反应。

更新日期:2019-05-09

中文翻译:

通过亲核取代和钯催化的交叉偶联策略将异恶唑-5-酮转化为3,5-二取代的异恶唑的通用平台
通过两步策略,已经开发出了将异恶唑-5-酮转化为3,5-二取代异恶唑的通用平台。第一步导致5-(假)卤代异恶唑的形成,而第二步则可以通过亲核取代或钯安装各种杂烷基链,杂芳基链,烷基链,烯基链,炔基链和芳基链催化的交叉偶联反应。
































 京公网安备 11010802027423号
京公网安备 11010802027423号