当前位置:
X-MOL 学术
›
Microchim. Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper(II) ions enhance the peroxidase-like activity and stability of keratin-capped gold nanoclusters for the colorimetric detection of glucose
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-04-08 , DOI: 10.1007/s00604-019-3395-8
Shuyi Ma , Jinjie Wang , Guang Yang , Jingxia Yang , Derun Ding , Min Zhang
Microchimica Acta ( IF 5.3 ) Pub Date : 2019-04-08 , DOI: 10.1007/s00604-019-3395-8
Shuyi Ma , Jinjie Wang , Guang Yang , Jingxia Yang , Derun Ding , Min Zhang
|
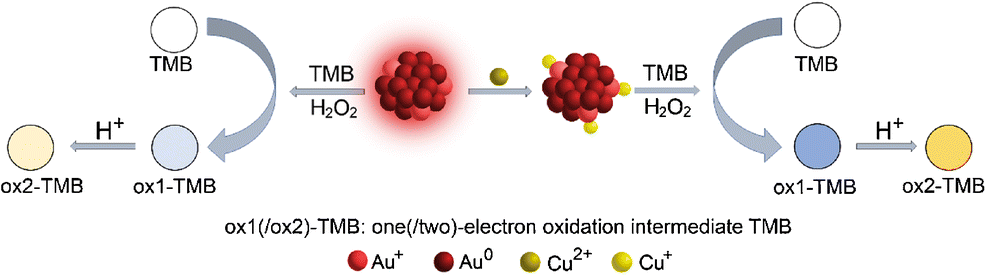
AbstractA method is described for the preparation of copper(II)-modified keratin-capped gold nanoclusters (AuNCs) with adjustable Au/Cu molar ratio through a two-step synthetic route. The introduction of Cu(II) is known to cause quenching of the fluorescence of such AuNCs. It is found, however, that the Cu(II) loaded AuNC (AuNC-Cu2+) display strongly enhanced peroxidase-like activity and improved chemical stability. This is assumed to be due to the synergistic effect of the gold and copper atoms and in contrast to the single components (pure AuNCs and copper ions). The kinetic parameters of the new peroxidase mimic show a higher Kcat value (12.1 × 10−4 s−1) and a lower Km value (53 μM) for H2O2 (compared to those of conventional AuNCs). The catalytic activity is stable and remains essentially unchanged after two months. The interactions of AuNCs with Cu(II) were characterized by fluorescence spectroscopy, UV-vis spectroscopy and X-ray photoelectron spectroscopy. Based on these findings, a glucose colorimetric assay at 452 nm was developed that has a detection range from 1.6 to 800 μM and a 0.26 μM detection limit. Graphical abstractCopper ion-modified keratin-capped gold nanoclusters (AuNC-Cu2+) exhibit enhanced peroxidase-like activity owing to the synergistic effect of the gold and copper atoms which is in contrast to pure AuNCs.
中文翻译:

铜(II)离子增强了角蛋白封端的金纳米团簇的过氧化物酶样活性和稳定性,用于葡萄糖的比色检测
摘要 描述了一种通过两步合成路线制备具有可调 Au/Cu 摩尔比的铜 (II) 修饰的角蛋白封端的金纳米团簇 (AuNCs) 的方法。已知引入 Cu(II) 会导致此类 AuNC 的荧光猝灭。然而,发现负载 Cu(II) 的 AuNC (AuNC-Cu2+) 显示出强烈增强的类过氧化物酶活性和改善的化学稳定性。这被认为是由于金和铜原子的协同作用,与单一成分(纯 AuNC 和铜离子)形成对比。新的过氧化物酶模拟物的动力学参数显示出更高的 Kcat 值(12.1 × 10-4 s-1)和更低的 H2O2 的 Km 值(53 μM)(与传统 AuNC 相比)。催化活性稳定,两个月后基本保持不变。AuNCs与Cu(II)的相互作用通过荧光光谱、紫外-可见光谱和X射线光电子能谱表征。基于这些发现,开发了 452 nm 的葡萄糖比色测定,其检测范围为 1.6 至 800 μM,检测限为 0.26 μM。图形摘要铜离子修饰的角蛋白封端的金纳米团簇 (AuNC-Cu2+) 由于金和铜原子的协同作用而表现出增强的过氧化物酶样活性,这与纯 AuNC 形成对比。
更新日期:2019-04-08
中文翻译:

铜(II)离子增强了角蛋白封端的金纳米团簇的过氧化物酶样活性和稳定性,用于葡萄糖的比色检测
摘要 描述了一种通过两步合成路线制备具有可调 Au/Cu 摩尔比的铜 (II) 修饰的角蛋白封端的金纳米团簇 (AuNCs) 的方法。已知引入 Cu(II) 会导致此类 AuNC 的荧光猝灭。然而,发现负载 Cu(II) 的 AuNC (AuNC-Cu2+) 显示出强烈增强的类过氧化物酶活性和改善的化学稳定性。这被认为是由于金和铜原子的协同作用,与单一成分(纯 AuNC 和铜离子)形成对比。新的过氧化物酶模拟物的动力学参数显示出更高的 Kcat 值(12.1 × 10-4 s-1)和更低的 H2O2 的 Km 值(53 μM)(与传统 AuNC 相比)。催化活性稳定,两个月后基本保持不变。AuNCs与Cu(II)的相互作用通过荧光光谱、紫外-可见光谱和X射线光电子能谱表征。基于这些发现,开发了 452 nm 的葡萄糖比色测定,其检测范围为 1.6 至 800 μM,检测限为 0.26 μM。图形摘要铜离子修饰的角蛋白封端的金纳米团簇 (AuNC-Cu2+) 由于金和铜原子的协同作用而表现出增强的过氧化物酶样活性,这与纯 AuNC 形成对比。































 京公网安备 11010802027423号
京公网安备 11010802027423号