Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Modification of Turpentine with Natural Chiral Preservation and Low-Risk Application Prospects in Crop Protection
ACS Omega ( IF 3.7 ) Pub Date : 2019-04-08 00:00:00 , DOI: 10.1021/acsomega.9b00241 Yanqing Gao , Jin Hao , Jian Li , Zhanqian Song 1 , Shibin Shang 1
ACS Omega ( IF 3.7 ) Pub Date : 2019-04-08 00:00:00 , DOI: 10.1021/acsomega.9b00241 Yanqing Gao , Jin Hao , Jian Li , Zhanqian Song 1 , Shibin Shang 1
Affiliation
|
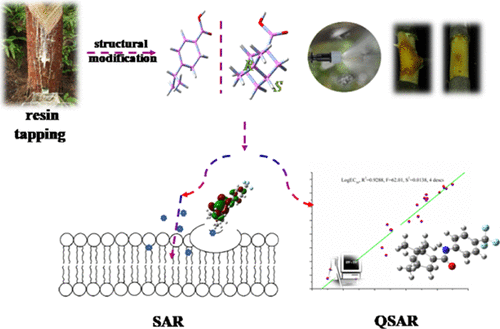
For the purpose of chiral pesticide development, the natural chiral structure of turpentine should be applied rationally. Two series of amide derivatives were prepared to study the effect of chiral center retention on the fungicidal activity. The investigation of fungicidal activity against three pathogenic microbes was carried out extensively. Some satisfactory conclusions were obtained from the activity evaluation. Above all, compounds (5′a-l) derived from cis-myrtlecanic acid (a chiral compound obtained from β-pinene) exhibited better activity than compounds (5a-l) derived from dehydrocumic acid (achiral compound obtained from β-pinene). The overall effect was good, and it was remarkable that compounds 5′d (substitute with N-(4-(trifluoromethyl)phenyl), 5′e (substitute with N-(4-fluorophenyl)), and 5′f (substitute with N-(4-chlorophenyl)) demonstrated extremely excellent activity, with EC50 values of 1.604, 1.822, and 2.296 μg/mL against Valsa mali. The above three compounds also showed a good control effect against V. mali in vitro on an apple branch. Treatment with 5′d, 5′e, and 5′f against V. mali resulted in significantly influenced physiological and biochemical indices, as well as markedly reduced pectinase activity in comparison with untreated controls. The preliminary structure–activity relationship (SAR) was summarized, showing that compounds obtained with chiral centers, a halogen atom, and small steric hindrance showed better performance. At the same time, the quantitative structure–activity relationship (QSAR) model (R2 = 0.9288, F = 62.01, S2 = 0.0138) was obtained, and two most important structural features were maximum net atomic charge for a F atom and shadow indices. Guided by this study, the highly efficient, low-toxicity, and environmentally friendly fungicide can be prepared through reasonable modification of terpene.
中文翻译:

天然手性防腐剂对松节油的结构修饰及低风险在作物保护中的应用前景
为了开发手性农药,应合理使用松节油的天然手性结构。制备了两个系列的酰胺衍生物以研究手性中心保留对杀真菌活性的影响。广泛地研究了对三种病原微生物的杀真菌活性。从活动评估中得出了一些令人满意的结论。最重要的是,衍生自顺式-肉豆蔻酸(获自β-pine烯的手性化合物)的化合物(5'al)比衍生自脱氢枯酸的化合物(5a-1)(获自β-pine烯的非手性化合物)表现出更好的活性。总体效果良好,值得注意的是化合物5'd(用N-(4-(三氟甲基)苯基)取代,化合物5'e(用N-(4-(三氟甲基)苯基)取代)N-(4-氟苯基))和5'f(用N-(4-氯苯基)替代)具有极好的活性,对Valsa mali的EC 50值为1.604、1.822和2.296μg / mL 。上述三种化合物在苹果分支上还表现出良好的体外防治马来弧菌的效果。治疗5'D,5'e,以及对5'F五马里与未处理的对照相比,可显着降低生理和生化指标的影响,并显着降低果胶酶的活性。总结了初步的结构-活性关系(SAR),表明具有手性中心,卤原子和小的位阻的化合物表现出更好的性能。同时,定量构效关系(QSAR)模型(R 2 = 0.9288,F = 62.01,S 2= 0.0138),两个最重要的结构特征是F原子的最大净原子电荷和阴影指数。在这项研究的指导下,可以通过合理修饰萜烯来制备高效,低毒和环境友好的杀菌剂。
更新日期:2019-04-08
中文翻译:

天然手性防腐剂对松节油的结构修饰及低风险在作物保护中的应用前景
为了开发手性农药,应合理使用松节油的天然手性结构。制备了两个系列的酰胺衍生物以研究手性中心保留对杀真菌活性的影响。广泛地研究了对三种病原微生物的杀真菌活性。从活动评估中得出了一些令人满意的结论。最重要的是,衍生自顺式-肉豆蔻酸(获自β-pine烯的手性化合物)的化合物(5'al)比衍生自脱氢枯酸的化合物(5a-1)(获自β-pine烯的非手性化合物)表现出更好的活性。总体效果良好,值得注意的是化合物5'd(用N-(4-(三氟甲基)苯基)取代,化合物5'e(用N-(4-(三氟甲基)苯基)取代)N-(4-氟苯基))和5'f(用N-(4-氯苯基)替代)具有极好的活性,对Valsa mali的EC 50值为1.604、1.822和2.296μg / mL 。上述三种化合物在苹果分支上还表现出良好的体外防治马来弧菌的效果。治疗5'D,5'e,以及对5'F五马里与未处理的对照相比,可显着降低生理和生化指标的影响,并显着降低果胶酶的活性。总结了初步的结构-活性关系(SAR),表明具有手性中心,卤原子和小的位阻的化合物表现出更好的性能。同时,定量构效关系(QSAR)模型(R 2 = 0.9288,F = 62.01,S 2= 0.0138),两个最重要的结构特征是F原子的最大净原子电荷和阴影指数。在这项研究的指导下,可以通过合理修饰萜烯来制备高效,低毒和环境友好的杀菌剂。






























 京公网安备 11010802027423号
京公网安备 11010802027423号