Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Twisted Ribbon Aggregates in a Model Peptide System.
Langmuir ( IF 3.7 ) Pub Date : 2019-04-08 00:00:00 , DOI: 10.1021/acs.langmuir.8b03886
Axel Rüter 1 , Stefan Kuczera 1 , Darrin J Pochan 2 , Ulf Olsson 1
Langmuir ( IF 3.7 ) Pub Date : 2019-04-08 00:00:00 , DOI: 10.1021/acs.langmuir.8b03886
Axel Rüter 1 , Stefan Kuczera 1 , Darrin J Pochan 2 , Ulf Olsson 1
Affiliation
|
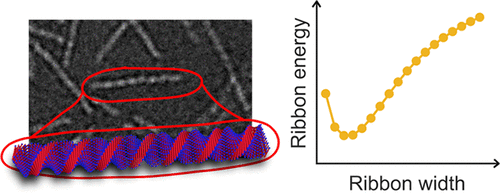
The model peptides A8K and A10K self-assemble in water into ca. 100 nm long ribbon-like aggregates. These structures can be described as β-sheets laminated into a ribbon structure with a constant elliptical cross-section of 4 by 8 nm, where the longer axis corresponds to a finite number, N ≈ 15, of laminated sheets, and 4 nm corresponds to a stretched peptide length. The ribbon cross-section is strikingly constant and independent of the peptide concentration. High-contrast transmission electron microscopy shows that the ribbons are twisted with a pitch λ ≈ 15 nm. The self-assembly is analyzed within a simple model taking into account the interfacial free energy of the hydrophobic β-sheets and a free energy penalty arising from an increased stretching of hydrogen bonds within the laminated β-sheets, arising from the twist of the ribbons. The model predicts an optimal value N, in agreement with the experimental observations.
中文翻译:

模型肽系统中的扭曲带状聚集体。
模型肽A 8 K和A 10 K在水中自组装为ca。100 nm长的带状聚集体。这些结构可以描述为β片层合成带状结构,具有恒定的椭圆形横截面4 x 8 nm,其中长轴对应于有限个数N层压片的≈15,且4 nm对应于拉伸的肽长度。条带横截面非常恒定,并且与肽浓度无关。高对比度透射电子显微镜显示,色带以λ≈15 nm的间距扭曲。在一个简单的模型中对自组装进行了分析,其中考虑了疏水性β-折叠层的界面自由能以及由于带的扭曲而导致的层压β-折叠层中氢键的拉伸增加导致的自由能损失。该模型与实验观察结果相符,可预测最佳值N。
更新日期:2019-04-08
中文翻译:

模型肽系统中的扭曲带状聚集体。
模型肽A 8 K和A 10 K在水中自组装为ca。100 nm长的带状聚集体。这些结构可以描述为β片层合成带状结构,具有恒定的椭圆形横截面4 x 8 nm,其中长轴对应于有限个数N层压片的≈15,且4 nm对应于拉伸的肽长度。条带横截面非常恒定,并且与肽浓度无关。高对比度透射电子显微镜显示,色带以λ≈15 nm的间距扭曲。在一个简单的模型中对自组装进行了分析,其中考虑了疏水性β-折叠层的界面自由能以及由于带的扭曲而导致的层压β-折叠层中氢键的拉伸增加导致的自由能损失。该模型与实验观察结果相符,可预测最佳值N。































 京公网安备 11010802027423号
京公网安备 11010802027423号