当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineering Fe–Fe3C@Fe–N–C Active Sites and Hybrid Structures from Dual Metal–Organic Frameworks for Oxygen Reduction Reaction in H2–O2 Fuel Cell and Li–O2 Battery
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-04-08 , DOI: 10.1002/adfm.201901531 Hao Wang 1, 2 , Feng‐Xiang Yin 3, 4 , Ning Liu 5 , Rong‐Hui Kou 6 , Xiao‐Bo He 3, 4 , Cheng‐Jun Sun 6 , Biao‐Hua Chen 5 , Di‐Jia Liu 2 , Hua‐Qiang Yin 7
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2019-04-08 , DOI: 10.1002/adfm.201901531 Hao Wang 1, 2 , Feng‐Xiang Yin 3, 4 , Ning Liu 5 , Rong‐Hui Kou 6 , Xiao‐Bo He 3, 4 , Cheng‐Jun Sun 6 , Biao‐Hua Chen 5 , Di‐Jia Liu 2 , Hua‐Qiang Yin 7
Affiliation
|
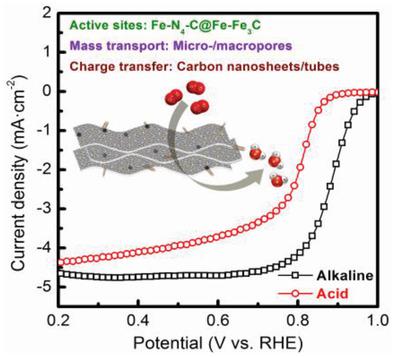
Dual metal–organic frameworks (MOFs, i.e., MIL‐100(Fe) and ZIF‐8) are thermally converted into Fe–Fe3C‐embedded Fe–N‐codoped carbon as platinum group metal (PGM)‐free oxygen reduction reaction (ORR) electrocatalysts. Pyrolysis enables imidazolate in ZIF‐8 rearranged into highly N‐doped carbon, while Fe from MIL‐100(Fe) into N‐ligated atomic sites concurrently with a few Fe–Fe3C nanoparticles. Upon precise control of MOF compositions, the optimal catalyst is highly active for the ORR in half‐cells (0.88 V in base and 0.79 V versus RHE in acid in half‐wave potential), a proton exchange membrane fuel cell (0.76 W cm−2 in peak power density) and an aprotic Li–O2 battery (8749 mAh g−1 in discharge capacity), representing a state‐of‐the‐art PGM‐free ORR catalyst. In the material, amorphous carbon with partial graphitization ensures high active site exposure and fast charge transfer simultaneously. Macropores facilitate mass transport to the catalyst surface, followed by oxygen penetration in micropores to reach the infiltrated active sites. Further modeling simulations shed light on the true Fe–Fe3C contribution to the catalyst performance, suggesting Fe3C enhances oxygen affinity, while metallic Fe promotes *OH desorption as the rate‐determining step at the nearby Fe–N–C sites. These findings demonstrate MOFs as model system for rational design of electrocatalyst for energy‐based functional applications.
中文翻译:

从H2–O2燃料电池和Li–O2电池中的氧还原反应中的双金属有机骨架工程化Fe–Fe3C @ Fe–N–C活性位点和混合结构
将双金属有机框架(MOF,即MIL-100(Fe)和ZIF-8)热转化为Fe-Fe 3 C嵌入的Fe-N掺杂碳,作为无铂族金属(PGM)的氧还原反应(ORR)电催化剂。热解使ZIF-8中的咪唑化物重新排列成高度掺杂N的碳,同时将MIL-100(Fe)中的Fe与一些Fe–Fe 3 C纳米粒子同时引入N连接的原子位。在MOF组合物的精确控制,最佳催化剂是对于在半电池的ORR(0.88 V在碱,并在半波电势酸0.79 V相对于RHE),质子交换膜燃料电池(0.76 w ^厘米高活性- 2个峰值功率密度)和非质子型Li–O 2电池(8749 mAh g -1放电容量),代表了最先进的无PGM的ORR催化剂。在这种材料中,具有部分石墨化作用的无定形碳可确保高活性位点暴露和快速的电荷转移。大孔有利于质量转移到催化剂表面,然后氧渗透到微孔中以到达渗透的活性位点。进一步的模拟模拟揭示了Fe–Fe 3 C对催化剂性能的真正贡献,表明Fe 3 C增强了氧亲和力,而金属Fe促进了* OH的解吸,这是附近Fe–N–C位置确定速率的步骤。这些发现证明,MOF是用于基于能量的功能应用的电催化剂合理设计的模型系统。
更新日期:2019-04-08
中文翻译:

从H2–O2燃料电池和Li–O2电池中的氧还原反应中的双金属有机骨架工程化Fe–Fe3C @ Fe–N–C活性位点和混合结构
将双金属有机框架(MOF,即MIL-100(Fe)和ZIF-8)热转化为Fe-Fe 3 C嵌入的Fe-N掺杂碳,作为无铂族金属(PGM)的氧还原反应(ORR)电催化剂。热解使ZIF-8中的咪唑化物重新排列成高度掺杂N的碳,同时将MIL-100(Fe)中的Fe与一些Fe–Fe 3 C纳米粒子同时引入N连接的原子位。在MOF组合物的精确控制,最佳催化剂是对于在半电池的ORR(0.88 V在碱,并在半波电势酸0.79 V相对于RHE),质子交换膜燃料电池(0.76 w ^厘米高活性- 2个峰值功率密度)和非质子型Li–O 2电池(8749 mAh g -1放电容量),代表了最先进的无PGM的ORR催化剂。在这种材料中,具有部分石墨化作用的无定形碳可确保高活性位点暴露和快速的电荷转移。大孔有利于质量转移到催化剂表面,然后氧渗透到微孔中以到达渗透的活性位点。进一步的模拟模拟揭示了Fe–Fe 3 C对催化剂性能的真正贡献,表明Fe 3 C增强了氧亲和力,而金属Fe促进了* OH的解吸,这是附近Fe–N–C位置确定速率的步骤。这些发现证明,MOF是用于基于能量的功能应用的电催化剂合理设计的模型系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号