Nature Communications ( IF 14.7 ) Pub Date : 2019-04-08 , DOI: 10.1038/s41467-019-09563-6
Liang Wei , Qiao Zhu , Lu Xiao , Hai-Yan Tao , Chun-Jiang Wang
|
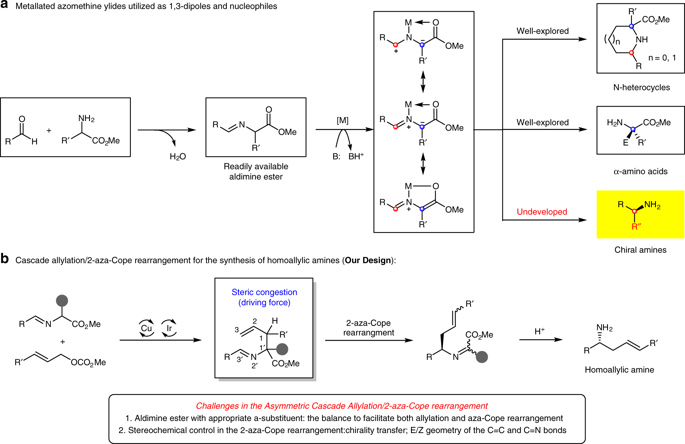
The efficient construction of enantiomerically enriched molecules from simple starting materials via catalytic asymmetric synthesis strategies is a key challenge in synthetic chemistry. Metallated azomethine ylides are commonly-used synthons for the preparation of N-heterocycles and α-amino acids. Remarkably, to date, the utilization of azomethine ylides for the facile access to chiral amines has proven elusive. Here, we report that a synergistic Cu/Ir-catalytic system combined with careful tuning of the steric congestion can be used to convert aldimine esters to a variety of chiral homoallylic amines via a cascade allylation/2-aza-Cope rearrangement. The elucidation of the distinct effects of each stereogenic center of the allylation intermediates on the stereochemical outcome and chirality transfer in the rearrangement further guided the selection of catalysts combination.
中文翻译:

协同催化偶氮甲亚胺的级联烯丙基化和2-氮杂应付
通过催化不对称合成策略从简单的起始原料有效构建对映异构体富集的分子是合成化学中的关键挑战。金属化的甲亚胺烷基化物是用于制备N-杂环和α-氨基酸的常用合成子。值得注意的是,迄今为止,已证明难以使用偶氮甲亚胺来容易地获得手性胺。在这里,我们报告协同的Cu / Ir催化系统与对空间拥堵的仔细调整相结合,可用于通过级联烯丙基化/ 2-氮杂-Cope重排将醛亚胺酯转化为多种手性均烯丙基胺。































 京公网安备 11010802027423号
京公网安备 11010802027423号