当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
What Triggers Oxygen Loss in Oxygen Redox Cathode Materials?
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-04-05 00:00:00 , DOI: 10.1021/acs.chemmater.9b00227
Robert A. House 1 , Urmimala Maitra 1 , Liyu Jin 1 , Juan G. Lozano 1 , James W. Somerville 1 , Nicholas H. Rees , Andrew J. Naylor 2 , Laurent C. Duda 3 , Felix Massel 3 , Alan V. Chadwick 4 , Silvia Ramos 4 , David M. Pickup 4 , Daniel E. McNally 5 , Xingye Lu 5 , Thorsten Schmitt 5 , Matthew R. Roberts 1 , Peter G. Bruce 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-04-05 00:00:00 , DOI: 10.1021/acs.chemmater.9b00227
Robert A. House 1 , Urmimala Maitra 1 , Liyu Jin 1 , Juan G. Lozano 1 , James W. Somerville 1 , Nicholas H. Rees , Andrew J. Naylor 2 , Laurent C. Duda 3 , Felix Massel 3 , Alan V. Chadwick 4 , Silvia Ramos 4 , David M. Pickup 4 , Daniel E. McNally 5 , Xingye Lu 5 , Thorsten Schmitt 5 , Matthew R. Roberts 1 , Peter G. Bruce 1
Affiliation
|
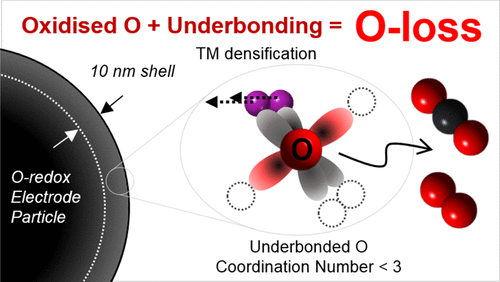
It is possible to increase the charge capacity of transition-metal (TM) oxide cathodes in alkali-ion batteries by invoking redox reactions on the oxygen. However, oxygen loss often occurs. To explore what affects oxygen loss in oxygen redox materials, we have compared two analogous Na-ion cathodes, P2-Na0.67Mg0.28Mn0.72O2 and P2-Na0.78Li0.25Mn0.75O2. On charging to 4.5 V, >0.4e– are removed from the oxide ions of these materials, but neither compound exhibits oxygen loss. Li is retained in P2-Na0.78Li0.25Mn0.75O2 but displaced from the TM to the alkali metal layers, showing that vacancies in the TM layers, which also occur in other oxygen redox compounds that exhibit oxygen loss such as Li[Li0.2Ni0.2Mn0.6]O2, are not a trigger for oxygen loss. On charging at 5 V, P2-Na0.78Li0.25Mn0.75O2 exhibits oxygen loss, whereas P2-Na0.67Mg0.28Mn0.72O2 does not. Under these conditions, both Na+ and Li+ are removed from P2-Na0.78Li0.25Mn0.75O2, resulting in underbonded oxygen (fewer than 3 cations coordinating oxygen) and surface-localized O loss. In contrast, for P2-Na0.67Mg0.28Mn0.72O2, oxygen remains coordinated by at least 2 Mn4+ and 1 Mg2+ ions, stabilizing the oxygen and avoiding oxygen loss.
中文翻译:

是什么引发氧气氧化还原阴极材料中的氧气损失?
通过在氧气上进行氧化还原反应,可以增加碱离子电池中过渡金属(TM)氧化物阴极的充电容量。但是,经常会发生氧气流失。为了探索什么影响氧氧化还原材料中的氧损失,我们比较了两个类似的Na离子阴极:P2-Na 0.67 Mg 0.28 Mn 0.72 O 2和P2-Na 0.78 Li 0.25 Mn 0.75 O 2。上充电至4.5V,> 0.4 ë -从这些材料的氧化物离子除去,但既不化合物显示出氧的损失。Li保留在P2-Na中0.78 Li 0.25 Mn 0.75 O从图2可以看到,但是从TM转移到碱金属层,表明TM层中的空位(也发生在其他表现出氧流失的氧氧化还原化合物中,例如Li [Li 0.2 Ni 0.2 Mn 0.6 MnO 2 ] O 2)不是触发因素。减少氧气。在5 V充电时,P2-Na 0.78 Li 0.25 Mn 0.75 O 2表现出氧损失,而P2-Na 0.67 Mg 0.28 Mn 0.72 O 2则没有氧损失。在这些条件下,Na +和Li +均从P2-Na中去除0.78 Li 0.25Mn 0.75 O 2,导致欠键合的氧(少于3个阳离子配位的氧)和表面局部O损失。相反,对于P2-Na 0.67 Mg 0.28 Mn 0.72 O 2,氧保持至少2 Mn 4+和1 Mg 2+离子的配位,稳定了氧并避免了氧的损失。
更新日期:2019-04-05
中文翻译:

是什么引发氧气氧化还原阴极材料中的氧气损失?
通过在氧气上进行氧化还原反应,可以增加碱离子电池中过渡金属(TM)氧化物阴极的充电容量。但是,经常会发生氧气流失。为了探索什么影响氧氧化还原材料中的氧损失,我们比较了两个类似的Na离子阴极:P2-Na 0.67 Mg 0.28 Mn 0.72 O 2和P2-Na 0.78 Li 0.25 Mn 0.75 O 2。上充电至4.5V,> 0.4 ë -从这些材料的氧化物离子除去,但既不化合物显示出氧的损失。Li保留在P2-Na中0.78 Li 0.25 Mn 0.75 O从图2可以看到,但是从TM转移到碱金属层,表明TM层中的空位(也发生在其他表现出氧流失的氧氧化还原化合物中,例如Li [Li 0.2 Ni 0.2 Mn 0.6 MnO 2 ] O 2)不是触发因素。减少氧气。在5 V充电时,P2-Na 0.78 Li 0.25 Mn 0.75 O 2表现出氧损失,而P2-Na 0.67 Mg 0.28 Mn 0.72 O 2则没有氧损失。在这些条件下,Na +和Li +均从P2-Na中去除0.78 Li 0.25Mn 0.75 O 2,导致欠键合的氧(少于3个阳离子配位的氧)和表面局部O损失。相反,对于P2-Na 0.67 Mg 0.28 Mn 0.72 O 2,氧保持至少2 Mn 4+和1 Mg 2+离子的配位,稳定了氧并避免了氧的损失。































 京公网安备 11010802027423号
京公网安备 11010802027423号